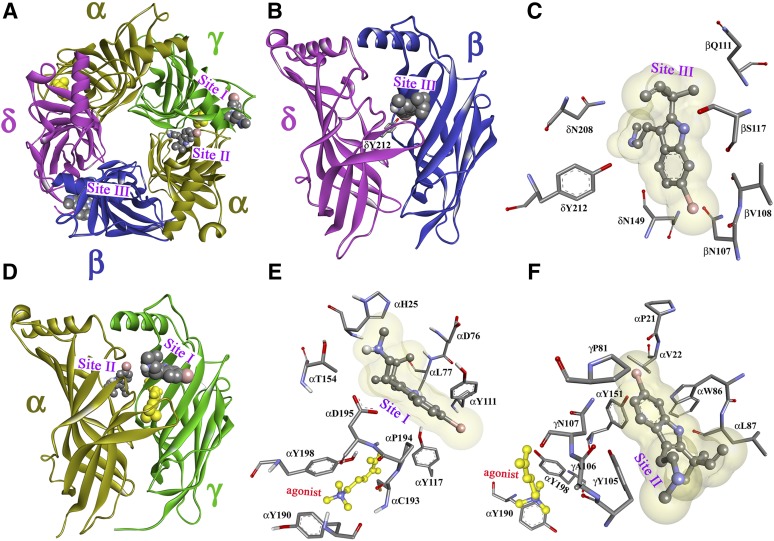
Fig. 11.
dFBr binding sites in the Torpedo nAChR ECD. Views of the homology model, the Torpedo nAChR ECD based on the x-ray structure of the L-AChBP in the presence of Carb (PDB ID 1UV6) (Celie et al., 2004) with the α, β, γ, and δ subunits colored in gold, blue, green, and violet, respectively. A view from the top (A) and side views from the exterior (B and D) are shown with dFBr, in space-filling representation, docked at three distinct sites in the presence of the agonist Carb (yellow). Sites I and II are at the agonist-binding canonical interface near entry to the ACh binding site (Site I; A, D, and E) and in the vestibule of the ion channel (Site II; A, D, and F). Site III (A, B, and C) is at the δ-β subunit interface. Views from the exterior (C and E) and a view from the ion channel vestibule (F) showing dFBr docked in its predicted lowest energy orientations in Site I (E), Site II (F), and Site III (C).