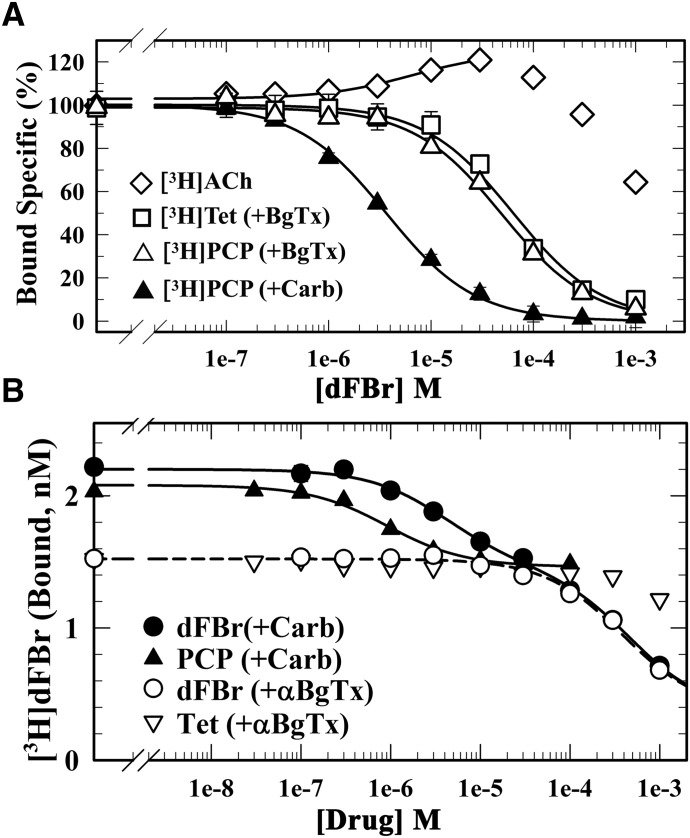
Fig. 4.
dFBr binds with high affinity to a site in the nAChR ion channel (desensitized state). The effects of dFBr on the equilibrium binding of [3H]ACh, [3H]PCP, and [3H]tetracaine (A) and the effect of dFBr, PCP, and tetracaine on the equilibrium binding of [3H]dFBr (B) to the Torpedo nAChR in the presence of α-BgTx (□, ▽, △) or Carb (⬤, ▴) were determined using centrifugation assays. (A) For each dFBr concentration, the specific binding of the radioligand was calculated from the total and nonspecific binding, normalized to the specific binding in the absence of dFBr, and fit to a single-site model using eq. 3. dFBr enhanced [3H]ACh binding (125% at 30 µM), inhibited [3H]PCP binding (+Carb) with IC50 = 3.6 ± 0.2 µM, and inhibited [3H]PCP and [3H]tetracaine binding in the presence of α-BgTx with IC50 values of 49 ± 6 and 63 ± 11 µM, respectively. (B) For each drug concentration, bound [3H]dFBr (in cpm/ml) was converted to nM [3H]dFBr. Inhibition curves were fit to a single-site model (eq. 1) or for inhibition by nonradioactive dFBr (+Carb) to a two-site model (eq. 2). For PCP (+Carb), IC50 = 0.9 ± 0.1 µM and Bns = 1.4 ± 0.1 nM, respectively. For dFBr (+α-BgTX), IC50 = 380 ± 70 µM, Bspec = 1.1 ± 0.08 nM, and Bns = 0.36 ± 0.09 nM. For inhibition by dFBr (+Carb), the high-affinity component of binding was fixed as the total binding [(+Carb) − (+α-BgTx)], the low-affinity component was equal to Bspec(+α-BgTx), and Bns was the same as in the presence of α-BgTx. With these assumptions, the calculated IC50 values for the high- and low-affinity components of binding were 4.1 ± 0.4 and 460 ± 30 µM, respectively.