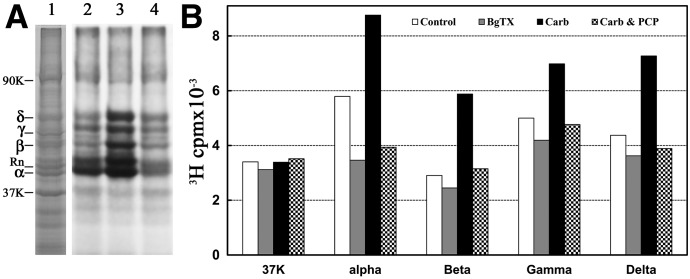
Fig. 5.
Photoincorporation of [3H]dFBr into Torpedo nAChR-rich membranes. Membranes were photolabeled on an analytical scale with 0.5 µM [3H]dFBr in the absence and presence of nAChR ligands. Polypeptides were resolved by SDS-PAGE on duplicate gels that were stained with Coomassie blue (A, lane 1). One gel was prepared for fluorography (A, lanes 2–4), and polypeptide bands were excised from the second gel for 3H determination by liquid scintillation counting (B). Drug additions for the fluorograph in (A): lane 2, no other ligand added; lane 3, +1 mM Carb; lane 4, +1 mM Carb and 100 µM PCP. The electrophoretic mobilities of the nAChR α, β, γ, and δ subunits, rapsyn (Rn), the Na+/K+-ATPase α subunit (90K) and calectrin (37K) are indicated on the left.