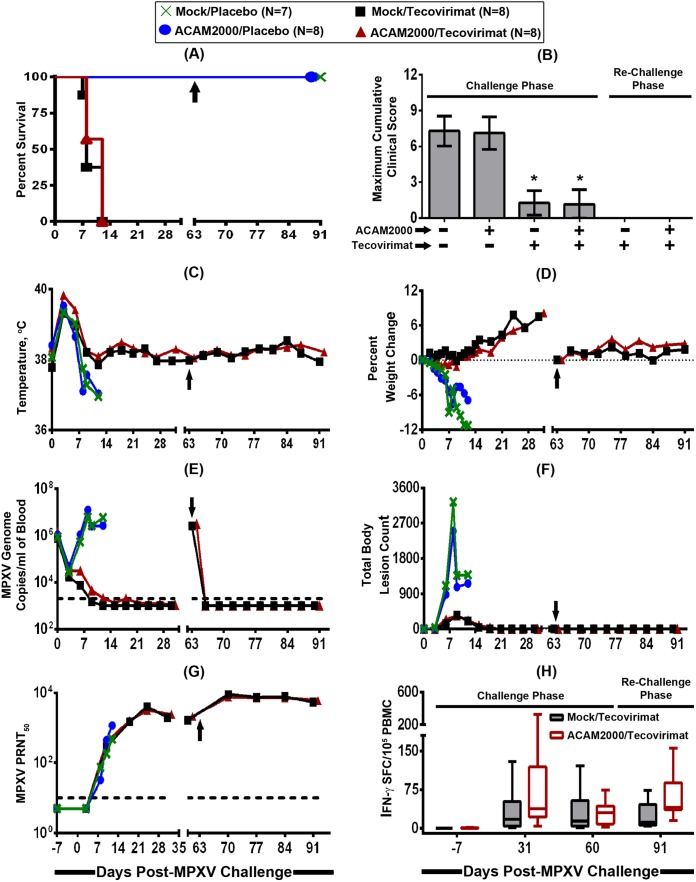
FIG 1.
Efficacy of smallpox antiviral tecovirimat given alone or in combination with smallpox vaccine ACAM2000 after MPXV exposure. Cynomolgus macaques (n = 7 or 8 animals/group) were intravenously infected with a target challenge dose of 5 × 107 PFU of MPXV Zaire-79 on study day 0 and vaccinated with vaccine diluent (mock vaccination) or ACAM2000 (one-time percutaneous scarification; 2.5 μl/2.5 × 105 to 12.5 × 105 PFU) on study day 3 and treated concurrently with a placebo or tecovirimat (10 mg/kg; oral gavage) once daily for 14 consecutive days (from study day 3 to day 16). The animals were then monitored for survival (A), clinical signs of disease (B), temperature (C) and weight (D) changes, viremia (E), body lesion formation (F), and development of humoral (G) and cellular (H) immune responses. Macaques that survived the initial MPXV challenge were rechallenged with a target challenge dose of 5 × 107 PFU of MPXV Zaire-79 2 months later (day 63; represented by an arrow) to assess for acquisition of immunological memory. Each curve in graphs B to G represents the arithmetic (B, C, D, and F) or geometric (E and G) mean value of the 7 or 8 animals in each treatment group. In order to avoid obscuring the mean data, the standard deviations are not shown (C to G). In panel H, the whiskers represent the minimum and the maximum values for the 7 or 8 animals in each treatment group. The dashed lines in panels E and G represent the lower limits of quantification for the assays. An asterisk (*) indicates statistical significance.