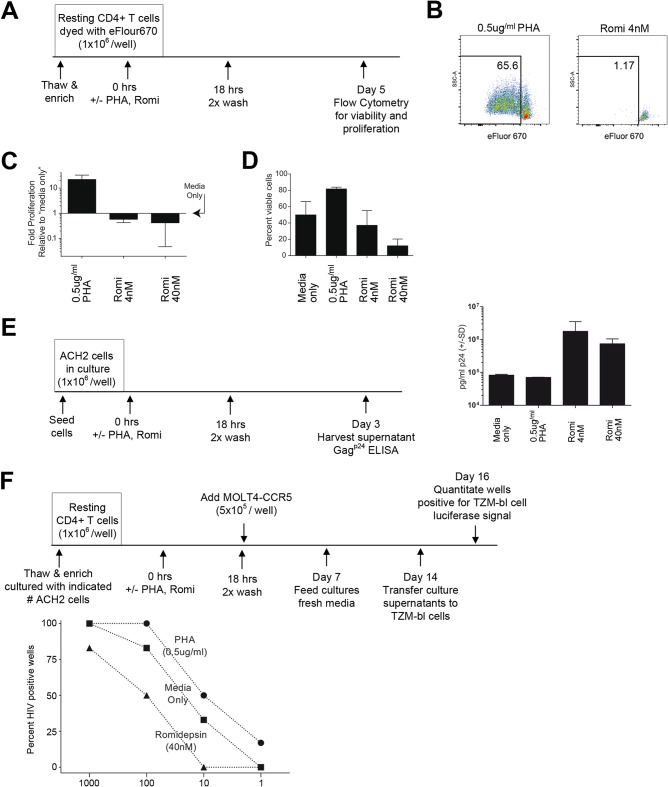
FIG 5.
Romidepsin decreases the proliferative capacity and viability of resting CD4+ T cells, leading to reduced sensitivity for the viral outgrowth assay. (A) Schematic representation of each experimental approach. (B to D) Flow cytometry data reveal that resting CD4+ T cell proliferation (B and C) and viability (D) are negatively impacted in response to romidepsin treatment. Viable cells exhibiting a dilution of eFluor-670 were defined as having proliferated during the culture period. The fold proliferation was determined relative to the respective human donor “medium only” culture from three human donors (means ± the SD) (C), and the percentage of viable cells represents the proportion of resting CD4+ T cells, which excluded the fixable green dead cell stain from three human donors (means ± the SD) (D). Error bars indicate the SD. (E) Culture supernatant Gagp24 quantities indicate that romidepsin, but not PHA, readily reactivates latent HIV in ACH2 cells (triplicate measures; means ± the SD). (F) Romidepsin treatment reduced the sensitivity of the viral outgrowth assay relative to medium control conditions (e.g., viral outgrowth from the condition recapitulating 100 IUPM was detected in 50% of the wells treated with romidepsin versus 83% for the medium control). Resting CD4+ T cells (106 per well) were cultured with the indicated numbers of ACH2 cells (n = 6 to 8 wells per data point [2 to 4 wells per human donor]). The results were analyzed by using a two-way ANOVA wherein the sources of variation were either the latency reactivation treatment (P = 0.0002) or the number of ACH2 cells per well (P = 0.012).