Abstract
Multidrug-resistant carbapenemase-producing Klebsiella pneumoniae (KpC) strains are becoming a common cause of infections in health care centers. Furthermore, Klebsiella can develop multicellular biofilms, which lead to elevated adaptive antibiotic resistance. Here, we describe the antimicrobial and antibiofilm activities of synthetic peptides DJK-5, DJK-6, and 1018 against five KpC isolates. Using static microplate assays, it was observed that the concentration required to prevent biofilm formation by these clinical isolates was below the MIC for planktonic cells. More-sophisticated flow cell experiments confirmed the antibiofilm activity of the peptides against 2-day-old biofilms of different KpC isolates, and in some cases, the peptides induced significant biofilm cell death. Clinically relevant combinations of DJK-6 and β-lactam antibiotics, including the carbapenem meropenem, also prevented planktonic growth and biofilm formation of KpC strain1825971. Interestingly, peptide DJK-6 was able to enhance, at least 16-fold, the ability of meropenem to eradicate preformed biofilms formed by this strain. Using peptide DJK-6 to potentiate the activity of β-lactams, including meropenem, represents a promising strategy to treat infections caused by KpC isolates.
INTRODUCTION
Klebsiella pneumoniae is a Gram-negative, encapsulated, opportunistic pathogen of the family Enterobacteriaceae (1). It has become a significant cause of infections, especially pneumonia, but also meningitis, sepsis, and urinary tract, device-associated, and surgical wound site infections (2–7). The most severe clinical cases of such infections are often associated with K. pneumoniae strains that produce a plasmid-borne carbapenemase (KpC).
The rate of fatality from KpC-producing K. pneumoniae is high (>50%) among patients with bloodstream infection (2, 8, 9).The treatment of these infections is difficult since KpC strains are resistant to all β-lactam antibiotics, including carbapenems, which are typically used as a last resort (10). In addition, bacteria harboring carbapenemase genes are often resistant to other antibiotics. As a consequence of this multidrug resistance, failure in treatment contributes to increased rates of morbidity and mortality (9, 11, 12). Tigecycline, aminoglycosides, and the polymyxins (polymyxin B and colistin) are currently the best treatment options for carbapenemase-producing strains (13). However, these strains also have the potential to develop resistance to these antibiotics (14–16).
The treatment of infections caused by multidrug-resistant bacteria is a challenge, and the ability of bacterial pathogens to form biofilms can further complicate this scenario. Biofilms, a distinct microbial lifestyle of bacteria, are surface-associated multicellular communities of bacteria encased in a protective polymeric matrix that can include polysaccharides, proteins, and DNA (17). They represent a substantial problem in the clinic, as they can lead to the development of chronic long-lived infections on body surfaces, including the lung, skin, heart, and bones (18–21), and can also be formed on medical devices (central venous catheters, urinary tract catheters, mechanical heart valve, contact lenses, endotracheal tubes, and implants) (18, 22–25).
Biofilms are highly (adaptively) resistant to antibiotics as well as to removal by host immune defense mechanisms and thus are very difficult to eradicate compared to their planktonic (free-swimming) counterparts, leading to increased health care costs (26). Although biofilms have a significant impact on public health, most research to date has focused on developing natural or synthetic antimicrobial agents that inhibit planktonic bacteria. Cationic amphipathic antimicrobial peptides have emerged in recent years as an alternative strategy for the treatment of antibiotic-resistant bacteria (27). Other so-called host defense peptides function as immunomodulatory agents with anti-infective, anti-inflammatory, and wound-healing properties, independent of direct antimicrobial activity (27).
Recently, it was shown that some cationic amphipathic peptides could prevent and/or eradicate bacterial biofilms in a manner that was independent of direct antimicrobial activity against free-swimming bacteria (28–30). Other studies demonstrated that the antibiofilm peptides 1018 (a synthetic variant of host defense peptides) and DJK-5 and DJK-6 (both designed D-enantiomeric protease-resistant peptides) had the ability to work synergistically with several conventional antibiotics, thus uncovering a novel approach to potentiating antibiotic action against bacterial biofilms (31, 32). Based on these studies, we hypothesized that the small cationic antibiofilm peptides 1018, DJK-5, and DJK-6 could both prevent biofilm formation and target preformed biofilms formed by carbapenemase-producing K. pneumoniae. The objective of this study was to investigate the biofilm-forming ability of KpC clinical strains isolated from patients at Brasilia Hospital and to test the activity of antibiofilm peptides against these isolates.
MATERIALS AND METHODS
Bacterial strains.
Clinical isolates of K. pneumoniae were obtained from hospitalized patients in Brasilia, Brazil, between 2010 and 2012. The isolate 1825971 was obtained from an endotracheal aspirate, and the isolates 1789769, 2144392, 2210477, and 1450421 were obtained from blood. The isolates were suspended in Mueller-Hinton broth and incubated for 18 to 24 h at 36°C. They were confirmed as Klebsiella pneumoniae strains using the MicroScan WalkAway automated system (Siemens Healthcare Diagnostics) according to the manufacturer's instructions. Standard biochemical tests were also used to confirm the species (33). Isolates were preserved at −70°C with the addition of 8% dimethyl sulfoxide.
Peptide synthesis.
DJK-5 (VQWRAIRVRVIR-NH2) and DJK-6 (VQWRRIRVWVIR-NH2), both D-enantiomeric peptides, and the peptide 1018 (VRLIVAVRIWRR-NH2) were synthesized by CPC Scientific using solid-phase 9-fluorenylmethoxy carbonyl (Fmoc) chemistry and purified to 95% using reverse-phase high-performance liquid chromatography (HPLC). Peptide mass was confirmed by mass spectrometry.
Antibiotic susceptibility of K. pneumoniae clinical isolates, screening for carbapenemases, and detection of blaKPC genes.
Susceptibility testing was performed using the MicroScan WalkAway automated method. The susceptibility breakpoints were interpreted in accordance to the CLSI protocols (34). Resistance to carbapenem antibiotics was due to the production of a carbapenemase, which was detected using the modified Hodge test (35). K. pneumoniae ATCC 700603 was used as a negative control, and K. pneumoniae IOC (a carbapenemase-producing strain from the culture collection of the Oswaldo Cruz Institute, Rio de Janeiro, Brazil) was used as a positive control. Specific primers were used under standard PCR conditions to detect and confirm the presence of genes encoding K. pneumoniae carbapenemase, blaKPC genes (F, 5′-TGTCACTGTATCGCCGTC and R, 5′-CTCAGTGCTCTACAGAAAACC) (36). The strains used as controls for the modified Hodge test were also used as negative and positive controls for PCR.
Biofilm formation and MIC of planktonic cells and biofilms.
K. pneumoniae biofilm formation was assessed both in rich media, i.e., Luria-Bertani (LB) broth (BD Difco) and Todd-Hewitt broth (BD Difco), and in BM2 minimal medium [62 mM potassium phosphate buffer, pH 7.0, 7 mM (NH4)2SO4, 2 mM MgSO4, 10 μM FeSO4, 0.5% glucose]. Overnight cultures of K. pneumoniae strains grown in LB broth were diluted 1 in 100 in each of these media, and 100 μl per well was added into 96-well round-bottom microplates. Planktonic cell growth was assessed by measuring absorbance at 600 nm. Nonadherent planktonic cells were removed from microtiter wells and washed twice with deionized water. Adherent cells were stained with 0.01% crystal violet for 20 min. The microplate wells were washed twice with deionized water, air dried, and solubilized with 110 μl of 70% ethanol. Biofilm formation was assessed by measuring the absorbance at 595 nm using a microplate reader (Bio-Tek Instruments, Inc., United States). Three biological replicates were performed for each experiment involving eight technical replicates. After establishing the best conditions for biofilm growth, antimicrobial peptides were evaluated for their ability to inhibit biofilm formation. The microdilution method for cationic peptides (37) was used to determine the MIC for planktonic cells and the minimal biofilm inhibitory concentration (MBIC) to inhibit biofilm formation by 50% (MBIC50) or 100% (MBIC100).
Checkerboard assay.
Synergy between DJK-6 and various antibiotics was assessed by checkerboard titration in 96-well microplates, wherein the peptide was diluted along the rows of a microtiter tray and the antibiotics were diluted along the columns. Planktonic growth and biofilm formation were assessed as described above. The results were expressed as the fractional inhibitory concentration index (FIC), which is determined as follows: FIC = ([A]/MICA) + ([B]/MICB), where MICA and MICB are the MICs of antibiotic A and peptide B alone and [A] and [B] are the MICs of A and B when in combination. The FIC index was interpreted as follows: FIC ≤ 0.5, synergistic effect; 0.5 < FIC ≤ 1, additive effect; 1 < FIC ≤ 2, indifferent effect. The same approach was used to calculate biofilm inhibition in combination using the MBIC100 in place of the MIC. All FIC calculations were determined taking the next-lowest MBIC100 value (e.g., for >64 μg · ml−1, we used 128 μg · ml−1). The antibiotics used in this assay included imipenem (Merck), meropenem (Sigma-Aldrich), cefotaxime (Sigma-Aldrich), cefepime (Bristol), polymyxin B (Sigma-Aldrich), and colistin sulfate (Sigma-Aldrich).
Flow cell assays.
Flow cell experiments were performed to assess the effects of antimicrobial treatments on preformed biofilms as described previously (28).
RESULTS
Susceptibility of KpC to multiple antibiotics and biofilm formation assessments in different media.
It was observed that clinical isolates of K. pneumoniae from patients admitted to Brasilia hospitals were often resistant to multiple antibiotics (Table 1). All isolates were positive for carbapenemase production, according to the modified Hodge test. Further, genotyping of β-lactamase resistance by PCR and DNA sequencing showed that all isolates harbored blaKPC genes. We also assessed the ability of the KpC clinical isolates to form static biofilms in nutrient-rich media such as LB and Todd-Hewitt and in a nutrient-limited medium (i.e., BM2). These isolates produced increased biofilm biomass (optical density at 595 nm [OD595] range, >0.7 absorbance units [AU]) in BM2 medium compared to the biomass of those grown in nutrient-rich media like LB and Todd-Hewitt broth (Table 2). In contrast, using different media did not affect the planktonic growth of these strains (Table 2).
TABLE 1.
Susceptibility of KPC-producing K. pneumoniae isolates to different antibioticsa
| Antibiotic | MIC and susceptibility of KpC isolate: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1450421 |
1789769 |
1825971 |
2144392 |
2210477 |
||||||
| MIC | Interpretation | MIC | Interpretation | MIC | Interpretation | MIC | Interpretation | MIC | Interpretation | |
| Amikacin | ≤16 | S | ≤16 | S | 32 | I | ≤16 | S | 32 | I |
| Ampicillin | >16 | R | >16 | R | >16 | R | >16 | R | >16 | R |
| Ampicillin-sulbactam | >16/8 | R | >16/8 | R | >16/8 | R | >16/8 | R | >16/8 | R |
| Aztreonam | >16 | R | >16 | R | >16 | R | >16 | R | >16 | R |
| Cefepime | >16 | R | >16 | R | >16 | R | >16 | R | >16 | R |
| Cefotaxime | >32 | R | >32 | R | >32 | R | >32 | R | >32 | R |
| Cefotetan | >32 | S | >32 | R | >32 | R | 32 | I | 32 | R |
| Ceftazidime | >16 | R | >16 | R | 16 | R | 8 | I | >16 | R |
| Ceftriaxone | >32 | R | >32 | R | >32 | R | >32 | R | >32 | R |
| Cefuroxime | >16 | R | >16 | R | >16 | R | >16 | R | >16 | R |
| Cephalothin | >16 | R | >16 | R | >16 | R | >16 | R | >16 | R |
| Ciprofloxacin | >2 | R | >2 | R | >2 | R | >2 | R | >2 | R |
| Ertapenem | >4 | R | >4 | R | >4 | R | >4 | R | >4 | R |
| Gentamicin | >8 | R | >8 | R | >8 | R | >8 | R | >8 | R |
| Imipenem | >8 | R | >8 | R | >8 | R | >8 | R | >8 | R |
| Levofloxacin | >4 | R | ≤2 | S | 4 | I | >4 | R | >4 | R |
| Meropenem | >8 | R | >8 | R | >8 | R | 8 | R | >8 | R |
| Nitrofurantoin | >64 | R | >64 | R | 64 | I | >64 | R | >64 | R |
| Piperacillin | >64 | R | >64 | R | >64 | R | >64 | R | >64 | R |
| Piperacillin-tazobactam | >64 | R | >64 | R | >64 | R | >64 | R | >64 | R |
| Tetracycline | >8 | R | 8 | I | 8 | I | ≤4 | S | >8 | R |
| Tigecycline | ≤2 | S | ≤2 | S | ≤2 | S | ≤2 | S | ≤2 | S |
| Tobramycin | 8 | I | >8 | R | >8 | R | >8 | R | >8 | R |
| Trimethoprim-sulfamethoxazole | >2/38 | R | ≤2/38 | S | >2/38 | R | ≤2/38 | S | >2/38 | R |
S, susceptible; I, intermediary; R, resistant.
TABLE 2.
Assessment of biofilm formation of KPC-producing K. pneumoniae isolates in different mediaa
| KpC isolate | Growth (mean absorbance ± SD) in: |
|||||
|---|---|---|---|---|---|---|
| Luria broth |
Todd-Hewitt broth |
BM2 glucose |
||||
| Planktonic cells | Biofilm cells | Planktonic cells | Biofilm cells | Planktonic cells | Biofilm cells | |
| 1450421 | 1.29 ± 0.04 | 0.20 ± 0.05 | 1.48 ± 0.04 | 0.17 ± 0.03 | 1.17 ± 0.06 | 1.28 ± 0.11 |
| 1789769 | 1.18 ± 0.05 | 0.05 ± 0.03 | 1.53 ± 0.03 | 0.11 ± 0.02 | 1.22 ± 0.06 | 0.80 ± 0.07 |
| 1825971 | 1.30 ± 0.06 | 0.27 ± 0.06 | 1.51 ± 0.04 | 0.12 ± 0.02 | 1.15 ± 0.04 | 0.79 ± 0.02 |
| 2144392 | 1.20 ± 0.05 | 0.02 ± 0.01 | 1.52 ± 0.03 | 0.04 ± 0.02 | 1.16 ± 0.06 | 0.62 ± 0.04 |
| 2210477 | 1.25 ± 0.07 | 0.15 ± 0.03 | 1.46 ± 0.05 | 0.14 ± 0.06 | 1.13 ± 0.01 | 1.18 ± 0.04 |
Adherence to the surfaces of the wells of 96-well microtiter plates was examined. After incubation for 24 h at 37°C, the growth of planktonic cells was measured as the absorbance at 600 nm (A600), while biofilm cell growth was assessed at A595 after crystal violet staining and ethanol extraction.
Effects of small cationic antibiofilm peptides in the prevention and eradication of KpC biofilms.
To investigate the effects of the small synthetic cationic peptides 1018, DJK-6, and DJK-5 on planktonic growth of KpC isolates, we performed microdilution susceptibility assays using BM2 medium and peptides at concentrations ranging between 0.5 and 64 μg · ml−1. The five tested KpC isolates showed variable susceptibility to the peptides under the conditions tested (Table 3).
TABLE 3.
Evaluation of the antimicrobial activity of peptides against planktonic (MIC) and biofilm (MBIC) cells of KPC-producing K. pneumoniae isolates using microtiter assaysa
| KpC isolate | IDR 1018 |
DJK-5 |
DJK-6 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| MIC | MBIC50 | MBIC100 | MIC | MBIC50 | MBIC100 | MIC | MBIC50 | MBIC100 | |
| 1450421 | 32 | 16 | 32 | 8 | 4 | 8 | 16 | 8 | 16 |
| 1789769 | 32 | 4 | 32 | 16 | 4 | 8 | 8 | 2 | 4 |
| 1825971 | >64 | 2 | 64 | >64 | 32 | >64 | 64 | 4 | 32 |
| 2144392 | 32 | 1 | 4 | 32 | 1 | 4 | 8 | 1 | 2 |
| 2210477 | 32 | 8 | 32 | 16 | 4 | 8 | >64 | 8 | >64 |
Values are in μg · ml−1.
The peptides were quite ineffective versus planktonic cells with MICs of 8 to >64 μg · ml−1. In contrast, the peptides showed good antibiofilm activity versus Klebsiella with MBIC50 ranging from 1 to 32 μg · ml−1. The MBIC100, which reflects inhibition of both biofilm formation and nonspecific binding to the microtiter wells, was 2- to 32-fold higher than the MBIC50 but still often lower than the MIC. Among individual clinical isolates, there was some variability, with biofilms from strains 1450421,1825971, and 2210477 showing higher MBICs and strains 1789769 and 2144392 biofilms being more susceptible.
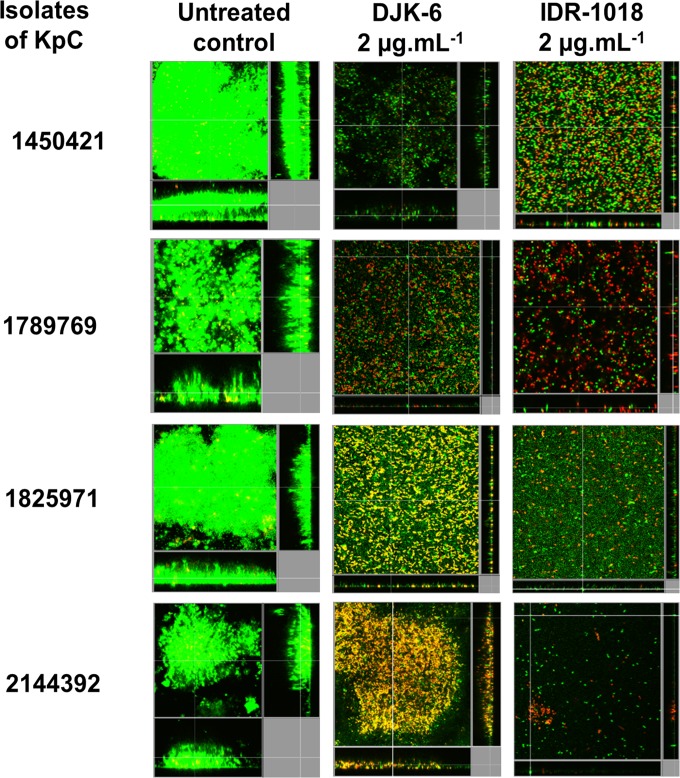
To test the ability of the antibiofilm peptides to eradicate preformed biofilms, we used the more accurate and sensitive flow cell method to test the susceptibility of biofilms of four KpC isolates (1450421, 1789769, 1825971, and 2144392) to peptides 1018 and DJK-6. Interestingly, strain 1450421 formed the most robust biofilms in our flow cell experiments (Fig. 1), consistent with its ability to form biofilms in microtiter plates (Table 2). The results for peptide addition to preformed biofilms revealed that at very low concentrations (2 μg · ml−1), the peptides 1018 and DJK-6 were able to disrupt and cause dispersal of 2-day-old biofilms of KpC isolates, reducing most preexisting biofilms to only a monolayer of cells or a few single cells attached to the surface of the flow cell chambers (Fig. 1). In most cases, the peptides triggered considerable cell death (yellow- and red-stained cells) among the cells remaining associated with the flow cell chambers, although the numbers of cells were considerably diminished, indicating also considerable dispersal. Intriguingly, the high sensitivity to peptides occurred even for isolate 1450421, which was considerably less susceptible in microtiter plate assays, potentially due to the high contribution of nonspecific binding to the microtiter plate surfaces in these assays.
FIG 1.
Effects of peptides 1018 and DJK-6 against preformed biofilms of KpC isolates. Biofilms were grown using the flow cell system. Treatments were applied on 2-day-old biofilms for 24 h. After this time, bacteria were stained green with the all-bacteria stain Syto-9 and red with the dead-bacteria stain propidium iodide (merge shows as yellow to red) prior to confocal imaging. Each panel shows reconstructions from the top in the large panel and sides in the right and bottom panels (xy, yz, and xz dimensions).
Synergy studies to prevent and eradicate KpC biofilms.
Since peptide DJK-6 exhibited high antibiofilm action against KpC isolates, this peptide was chosen for synergy studies using checkerboard titration assays with the multidrug-resistant isolate KpC1825971. Despite choosing antibiotics to which these KpC isolates were highly resistant (MICs and MBICs of >64 μg · ml−1), we observed that peptide DJK-6 enhanced the activity of the β-lactam antibiotics meropenem, imipenem, and cefepime, preventing biofilm growth of strain KpC 1825971 (Table 4). In contrast, the combinations between peptide DJK-6 and cefotaxime, polymyxin B, or colistin were unable to prevent biofilm formation by this Klebsiella strain.
TABLE 4.
Evaluation of the antimicrobial activity of conventional antibiotics in preventing planktonic (MIC) and biofilm (MBIC) growth of K. pneumoniae KpC strain 1825971 in microtiter assays and effects of the combination of peptide DJK-6 and the conventional antibiotics by checkerboard titration assessing the fractional inhibitory concentration index (FIC)a
| Antibiotic | MIC | MBIC50 | MBIC100 | MBIC of antibiotic in combination with DJK-6 | MBIC of DJK-6 in combination with antibiotic | FIC with peptide | Interpretationb | Fold reduction in antibiotic concn at the FIC |
|---|---|---|---|---|---|---|---|---|
| Meropenem | >64 | >64 | >64 | 4 | 12 | 0.4 | Syn | 32 |
| Imipenem | >64 | >64 | >64 | 8 | 12 | 0.4 | Syn | 16 |
| Cefepime | >64 | >64 | >64 | 8 | 12 | 0.4 | Syn | 16 |
| Cefotaxime | >64 | >64 | >64 | 64 | 32 | 1.5 | Ind | 2 |
| Colistin | >64 | >64 | >64 | 64 | 48 | 2.0 | Ind | 2 |
| Polymyxin | >64 | >64 | >64 | 64 | 48 | 2.0 | Ind | 2 |
All MIC and MBIC values are in μg · ml−1. FIC calculations were determined taking the next-lowest MBIC100 value (e.g., for >64 μg · ml−1, we used 128 μg · ml−1).
Syn, synergy; Ind, indifferent.
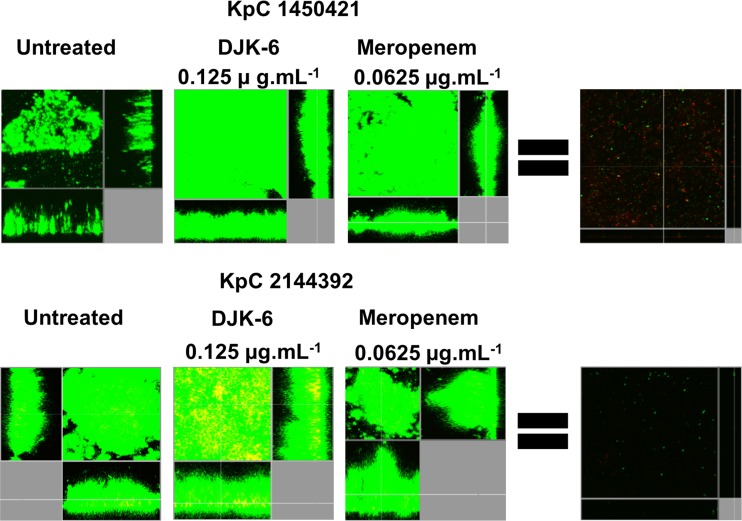
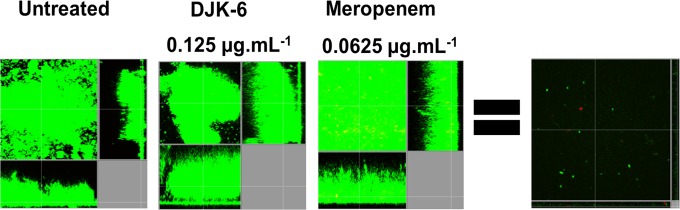
The finding of synergy with two carbapenem antibiotics was very surprising, especially given the presence of a highly active carbapenemase in this isolate. Therefore, we further explored the combination of peptide DJK-6 with meropenem to eradicate preformed flow cell biofilms of KpC 1825971. We determined the concentrations of both DJK-6 and meropenem, alone or combined, required to eradicate the majority of biofilm cells (DJK-6, 8 μg · ml−1; meropenem, 1 μg · ml−1) and those that did not affect preformed biofilms compared to the untreated control cells (DJK-6, 0.125 μg · ml−1; meropenem, 0.0625 μg · ml−1). The results revealed that the combination of only 0.125 μg · ml−1 of peptide DJK-6 and 0.0625 μg · ml−1 of meropenem cleared preexisting biofilms (Fig. 2), thus reducing at least 16-fold the concentration of meropenem required to clear biofilms formed by strain KpC 1825971. Other KpC isolates, 2144392 and 1450421, showed similar susceptibility to this combination of DJK-6 plus meropenem (Fig. 3).
FIG 2.
Effects of the combination of peptide DJK-6 and meropenem on preformed biofilms of KpC 1825971. Biofilms were grown in the flow cell system. Treatments were applied to 2-day-old biofilms for 24 h. After this time, bacteria were stained green with the all-bacteria stain Syto-9 and red with the dead-bacteria stain propidium iodide (merge shows as yellow to red) prior to confocal imaging. Each panel shows reconstructions from the top in the large panel and sides in the right and bottom panels (xy, yz, and xz dimensions).
FIG 3.
Effects of the combination of peptide DJK-6 and meropenem on preformed biofilms of KpC strains 1450421 and 2144392. Biofilms were grown in a flow cell system. Treatments were applied to a 2-day-old biofilm for 24 h. After this time, bacteria were stained green with the all-bacteria stain Syto-9 and red with the dead-bacteria stain propidium iodide (merge shows as yellow to red) prior to confocal imaging. Each panel shows reconstructions from the top in the large panel and sides in the right and bottom panels (xy, yz, and xz dimensions).
DISCUSSION
We found that clinical isolates of K. pneumoniae were resistant to multiple antibiotics, harbored KPC genes, and formed more biofilm in minimal medium than in nutrient broth. Previous studies showed that bacteria harboring KPC genes are resistant to several antibiotics, including the β-lactam antibiotics carbapenems, considered to be last-resort agents to treat serious infections caused by Gram-negative bacteria (13). The highest growth of biofilms in limited-nutrient medium in relation to nutrient-rich medium could be associated with the stress caused by limitation of one or more nutrients. For example, limitation of iron, magnesium, and lactose can induce a higher biofilm formation in Legionella pneumophila (38), Pseudomonas aeruginosa (39), and Citrobacter sp. (40), respectively.
Carbapenemase-producing strains represent a serious worldwide problem due to their multidrug resistance, easy dissemination, and limited available treatment options (41). Moreover, the potential of these strains to form biofilms on medical devices and body surfaces contributes to worsen this scenario. Many studies have shown that biofilm formation contributes to highly recalcitrant bacterial infections (20). However, few reports have focused on the development of new antimicrobials that target biofilms. Even more limited are studies focusing specifically on targeting carbapenemase-producing Enterobacteriaceae.
In our experiments, we investigated whether small synthetic peptides exhibited antibiofilm properties against KpC isolates. We observed that antibiofilm peptides 1018, DJK-6, and DJK-5 prevented biofilm formation of the different KpC strains tested (Table 3). In all cases, the concentration required to inhibit biofilm formation by 50% was lower than the MIC. Antibiofilm peptides, including peptide 1018, have demonstrated similar effects against other pathogenic bacteria (28, 29).
In experiments looking at the effects of the peptides on preformed flow cell biofilms (Fig. 1), we observed that both peptides 1018 and DJK-6 efficiently reduced biofilms to either a monolayer of cells or a few attached cells and many of the remaining cells were stained yellow or red, indicating that they were killed. These findings are consistent with recent studies showing that peptides 1018 (28) and DJK-6 (32) reduce biofilms of several bacteria, including K. pneumoniae. However, in our studies we used carbapenem-resistant K. pneumoniae strains instead of the carbapenem-susceptible strains used in those investigations.
Surprisingly, peptide DJK-6 was able to increase the antibiofilm activity of β-lactam antibiotics, including two carbapenems, in preventing biofilm formation (Table 4). Indeed, exceptionally small amounts of meropenem were required together with very low levels of peptide to completely eradicate preexisting biofilms of KpC (Fig. 2 and 3). Previous studies had indicated no other classes of antibiotics that were synergistic with DJK-6 (32). More specifically, the ability of DJK-6 to make biofilms of KpC isolates susceptible to carbapenems could be a potential approach to treat serious infections caused by carbapenem-resistant strains. In particular, this combination has the potential to treat biofilms formed on body surfaces, since it was able to clear biofilms grown in flow cells. These results confirm that small cationic antibiofilm peptides constitute a promising approach to increase the potency of conventional antibiotics against biofilms formed by resistant strains (32). Recent studies also showed that LL-37-derived peptides were able to eradicate existing biofilms of methicillin-resistant Staphylococcus aureus (MRSA) in thermally wounded human skin equivalents (42). Taken together, our results indicate that the combination of DJK-6 and meropenem could be a promising topical treatment strategy against infections caused by biofilms.
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R21AI098701 and by a grant from the Canadian Institutes for Health Research, MOP-74493. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. R.E.W.H. holds a Canada Research Chair in Health and Genomics. C.D.L.F.-N. received a scholarship from the Fundación “la Caixa” and Fundación Canadá (Spain). This work was also supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Pesquisa e Desenvolvimento (CNPq), Fundação de Amparo à Pesquisa do Distrito Federal (FAPDF) and Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul (FUNDECT). S.M.R. and O.L.F. received scholarships from CAPES/CNPq/Ciências em Fronteiras (Brazil).
Additionally, C.D.L.F.-N and R.E.W.H. are coinventors of a provisional patent application on the use of cationic antibiofilm and innate defense regulator (IDR) peptides (U.S. patent application no. 61/870,655).
REFERENCES
- 1.Podschun R, Ullmann U. 1998. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev 11:589–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pagano L, Caira M, Trecarichi EM, Spanu T, Di Blasi R, Sica S, Sanguinetti M, Tumbarello M. 2014. Carbapenemase-producing Klebsiella pneumoniae and hematologic malignancies. Emerg Infect Dis 20:1235–1236. doi: 10.3201/eid2007.130094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai Y, Zhang C, Ma X, Chang W, Hu S, Jia H, Huang J, Lu H, Li H, Zhou S, Qiu G, Liu J. 2014. Outbreak of carbapenemase-producing Klebsiella pneumoniae neurosurgical site infections associated with a contaminated shaving razor used for preoperative scalp shaving. Am J Infect Control 42:805–806. doi: 10.1016/j.ajic.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 4.Tuon FF, Rocha JL, Arend LN, Wallbach K, Zanin HA, Pilonetto M. 2013. Treatment and outcome of nine cases of KPC-producing Klebsiella pneumoniae meningitis. J Infect 67:161–164. doi: 10.1016/j.jinf.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 5.van Duin D, Cober E, Richter SS, Perez F, Kalayjian RC, Salata RA, Evans S, Fowler VG, Kaye KS, Bonomo RA. 2015. Impact of therapy and strain type on outcomes in urinary tract infections caused by carbapenem-resistant Klebsiella pneumoniae. J Antimicrob Chemother 70:1203–1211. doi: 10.1093/jac/dku495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatterjee S, Maiti P, Dey R, Kundu A, Dey R. 2014. Biofilms on indwelling urologic devices: microbes and antimicrobial management prospect. Ann Med Health Sci Res 4:100–104. doi: 10.4103/2141-9248.126612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song C, Yu J, Ai Q, Liu D, Lu W, Lu Q, Peng N. 2013. Diversity analysis of biofilm bacteria on tracheal tubes removed from intubated neonates. Zhonghua Er Ke Za Zhi 51:602–606. (In Chinese.) [PubMed] [Google Scholar]
- 8.Snitkin ES, Zelazny AM, Thomas PJ, Stock F, Henderson DK, Palmore TN, Segre JA. 2012. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci Transl Med 4:148ra116. doi: 10.1126/scitranslmed.3004129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borer A, Saidel-Odes L, Riesenberg K, Eskira S, Peled N, Nativ R, Schlaeffer F, Sherf M. 2009. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect Control Hosp Epidemiol 30:972–976. doi: 10.1086/605922. [DOI] [PubMed] [Google Scholar]
- 10.McKenna M. 2013. Antibiotic resistance: the last resort. Nature 499:394–396. doi: 10.1038/499394a. [DOI] [PubMed] [Google Scholar]
- 11.Gasink LB, Edelstein PH, Lautenbach E, Synnestvedt M, Fishman NO. 2009. Risk factors and clinical impact of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Infect Control Hosp Epidemiol 30:1180–1185. doi: 10.1086/648451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qureshi ZA, Paterson DL, Potoski BA, Kilayko MC, Sandovsky G, Sordillo E, Polsky B, Adams-Haduch JM, Doi Y. 2012. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother 56:2108–2113. doi: 10.1128/AAC.06268-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirsch EB, Tam VH. 2010. Detection and treatment options for Klebsiella pneumoniae carbapenemases (KPCs): an emerging cause of multidrug-resistant infection. J Antimicrob Chemother 65:1119–1125. doi: 10.1093/jac/dkq108. [DOI] [PubMed] [Google Scholar]
- 14.Spanu T, Angelis GD, Cipriani M, Pedruzzi B, D'Inzeo T, Cataldo MA, Sganga G, Tacconelli E. 2012. In vivo emergence of tigecycline resistance in multidrug-resistant Klebsiella pneumoniae and Escherichia coli. Antimicrob Agents Chemother 56:4516–4518. doi: 10.1128/AAC.00234-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kontopoulou K, Protonotariou E, Vasilakos K, Kriti M, Koteli A, Antoniadou E, Sofianou D. 2010. Hospital outbreak caused by Klebsiella pneumoniae producing KPC-2 β-lactamase resistant to colistin. J Hosp Infect 76:70–73. doi: 10.1016/j.jhin.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 16.Almaghrabi R, Clancy CJ, Doi Y, Hao B, Chen L, Shields RK, Press EG, Iovine NM, Townsend BM, Wagener MM, Kreiswirth B, Nguyen MH. 2014. Carbapenem-resistant Klebsiella pneumoniae strains exhibit diversity in aminoglycoside modifying enzymes, which exert varying effects on plazomicin and other agents. Antimicrob Agents Chemother 58:4443–4451. doi: 10.1128/AAC.00099-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de la Fuente-Núñez C, Reffuveille F, Fernández L, Hancock REW. 2013. Bacterial biofilm development as a multicellular adaptation: antibiotic resistance and new therapeutic strategies. Curr Opin Microbiol 16:580–589. doi: 10.1016/j.mib.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 18.Malizos KN, Ioannou M. 2014. Bone-implant interface in biofilm-associated bone and joint infections, p 239–253. In Karachalios T. (ed), Bone-implant interface in orthopedic surgery, 1st ed Springer, London, England. [Google Scholar]
- 19.Roy S, Elgharably H, Sinha M, Ganesh K, Chaney S, Mann E, Miller C, Khanna S, Bergdall VK, Powell HM, Cook CH, Gordillo GM, Wozniak DJ, Sen CK. 2014. Mixed-species biofilm compromises wound healing by disrupting epidermal barrier function. J Pathol 233:331–343. doi: 10.1002/path.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bjarnsholt T. 2013. The role of bacterial biofilms in chronic infections. APMIS Suppl 2013(136):1–51. [DOI] [PubMed] [Google Scholar]
- 21.Lanter BB, Sauer K, Davies DG. 2014. Bacteria present in carotid arterial plaques are found as biofilm deposits which may contribute to enhanced risk of plaque rupture. mBio 5:e01206–14. doi: 10.1128/mBio.01206-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung C-J, Yeh C-Y, Shun C-T, Hsu R-B, Cheng H-W, Lin C-S, Chia J-S. 2012. Platelets enhance biofilm formation and resistance of endocarditis-inducing streptococci on the injured heart valve. J Infect Dis 205:1066–1075. doi: 10.1093/infdis/jis021. [DOI] [PubMed] [Google Scholar]
- 23.Abidi SH, Sherwani SK, Siddiqui TR, Bashir A, Kazmi SU. 2013. Drug resistance profile and biofilm forming potential of Pseudomonas aeruginosa isolated from contact lenses in Karachi-Pakistan. BMC Ophthalmol 13:57. doi: 10.1186/1471-2415-13-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vandecandelaere I, Matthijs N, Van Nieuwerburgh F, Deforce D, Vosters P, De Bus L, Nelis HJ, Depuydt P, Coenye T. 2012. Assessment of microbial diversity in biofilms recovered from endotracheal tubes using culture dependent and independent approaches. PLoS One 7:e38401. doi: 10.1371/journal.pone.0038401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pour NK, Dusane DH, Dhakephalkar PK, Zamin FR, Zinjarde SS, Chopade BA. 2011. Biofilm formation by Acinetobacter baumannii strains isolated from urinary tract infection and urinary catheters. FEMS Immunol Med Microbiol 62:328–338. doi: 10.1111/j.1574-695X.2011.00818.x. [DOI] [PubMed] [Google Scholar]
- 26.Römling U, Kjelleberg S, Normark S, Nyman L, Uhlin BE, Åkerlund B. 2014. Microbial biofilm formation: a need to act. J Intern Med 276:98–110. doi: 10.1111/joim.12242. [DOI] [PubMed] [Google Scholar]
- 27.Fjell CD, Hiss JA, Hancock REW, Schneider G. 2012. Designing antimicrobial peptides: form follows function. Nat Rev Drug Discov 11:37–51. doi: 10.1038/nrd3591. [DOI] [PubMed] [Google Scholar]
- 28.de la Fuente-Núñez C, Reffuveille F, Haney EF, Straus SK, Hancock REW. 2014. Broad-spectrum anti-biofilm peptide that targets a cellular stress response. PLoS Pathog 10:e1004152. doi: 10.1371/journal.ppat.1004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de la Fuente-Núñez C, Korolik V, Bains M, Nguyen U, Breidenstein EBM, Horsman S, Lewenza S, Burrows L, Hancock REW. 2012. Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrob Agents Chemother 56:2696–2704. doi: 10.1128/AAC.00064-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Overhage J, Campisano A, Bains M, Torfs ECW, Rehm BHA, Hancock REW. 2008. Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect Immun 76:4176–4182. doi: 10.1128/IAI.00318-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reffuveille F, de la Fuente-Núñez C, Mansour S, Hancock REW. 2014. A broad-spectrum antibiofilm peptide enhances antibiotic action against bacterial biofilms. Antimicrob Agents Chemother 58:5363–5371. doi: 10.1128/AAC.03163-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de la Fuente-Núñez C, Reffuveille F, Mansour SC, Reckseidler-Zenteno SL, Hernández D, Brackman G, Coenye T, Hancock REW. 2015. D-Enantiomeric peptides that eradicate wild-type and multidrug-resistant biofilms and protect against lethal Pseudomonas aeruginosa infections. Chem Biol 22:196–205. doi: 10.1016/j.chembiol.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abbott SL. 2011. Klebsiella, Enterobacter, Citrobacter, Serratia, Plesiomonas, and other Enterobacteriaceae, p 639–657. In Versalovic J, Carroll K, Funke G, Jorgensen J, Landry M, Warnock D (ed), Manual of clinical microbiology, 10th ed ASM Press, Washington, DC. [Google Scholar]
- 34.Clinical and Laboratory Standards Institute. 2012. Methods for antimicrobial susceptibility testing of anaerobic bacteria. Approved standard—8th ed CLSI document M11-A8 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 35.Anderson KF, Lonsway DR, Rasheed JK, Biddle J, Jensen B, McDougal LK, Carey RB, Thompson A, Stocker S, Limbago B, Patel JB. 2007. Evaluation of methods to identify the Klebsiella pneumoniae carbapenemase in Enterobacteriaceae. J Clin Microbiol 45:2723–2725. doi: 10.1128/JCM.00015-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yigit H, Queenan AM, Anderson GJ, Domenech-Sanchez A, Biddle JW, Steward CD, Alberti S, Bush K, Tenover FC. 2001. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother 45:1151–1161. doi: 10.1128/AAC.45.4.1151-1161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiegand I, Hilpert K, Hancock REW. 2008. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc 3:163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- 38.Hindré T, Brüggemann H, Buchrieser C, Héchard Y. 2008. Transcriptional profiling of Legionella pneumophila biofilm cells and the influence of iron on biofilm formation. Microbiology 154:30–41. doi: 10.1099/mic.0.2007/008698-0. [DOI] [PubMed] [Google Scholar]
- 39.Mulcahy H, Lewenza S. 2011. Magnesium limitation is an environmental trigger of the Pseudomonas aeruginosa biofilm lifestyle. PLoS One 6:e23307. doi: 10.1371/journal.pone.0023307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allan VJM, Callow ME, Macaskie LE, Paterson-Beedle M. 2002. Effect of nutrient limitation on biofilm formation and phosphatase activity of a Citrobacter sp. Microbiology 148:277–288. [DOI] [PubMed] [Google Scholar]
- 41.Chen L, Chavda KD, Melano RG, Hong T, Rojtman AD, Jacobs MR, Bonomo RA, Kreiswirth BN. 2014. Molecular survey of the dissemination of two blaKPC-harboring IncFIA plasmids in New Jersey and New York hospitals. Antimicrob Agents Chemother 58:2289–2294. doi: 10.1128/AAC.02749-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haisma EM, Breij A de, Chan H, Dissel JT van, Drijfhout JW, Hiemstra PS, Ghalbzouri AE, Nibbering PH. 2014. LL-37-derived peptides eradicate multidrug-resistant Staphylococcus aureus from thermally wounded human skin equivalents. Antimicrob Agents Chemother 58:4411–4419. doi: 10.1128/AAC.02554-14. [DOI] [PMC free article] [PubMed] [Google Scholar]