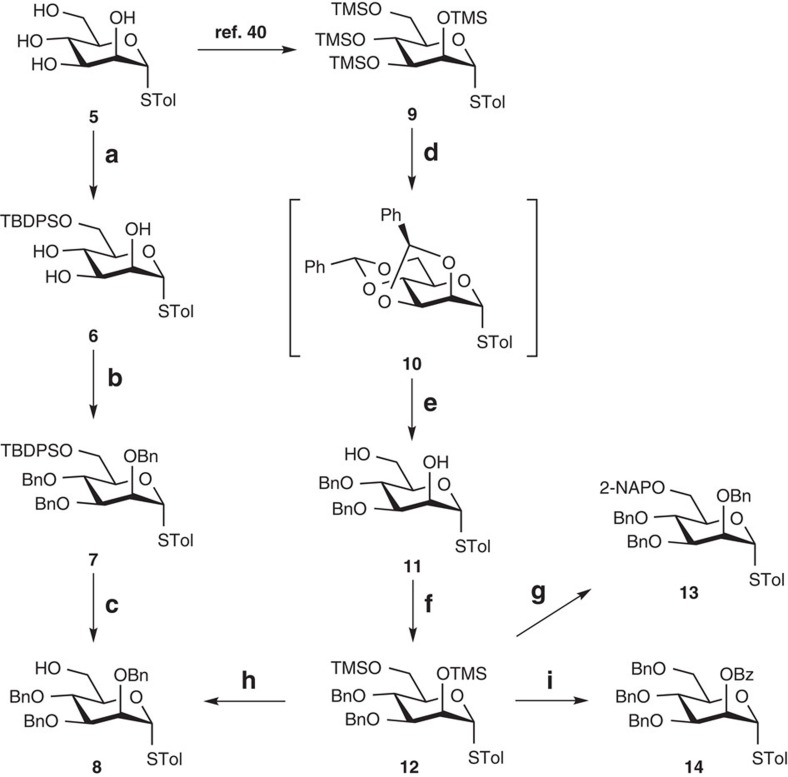
Figure 2. Preparations of the mannosyl-building blocks.
Reagents and conditions: (a) tert-butyldiphenylchlorosilane, Et3N, DMAP, 93%; (b) NaH, BnBr, DMF, 94%; (c) PTSA, MeOH, CH2Cl2, 92%; (d) benzaldehyde (2.1 equivalents), TMSOTf, MeCN, 0 °C, 30 min; (e) BH3·THF, Cu(OTf)2, CH2Cl2, 87% (one pot from 9); (f) trimethylchlorosilane, Et3N, quantitative; (g) 2-naphthaldehyde, Et3SiH, TMSOTf, CH2Cl2, −78 to −40 °C, 2 h, then, NaH, BnBr, DMF, 81% (one pot); (h) 2-naphthaldehyde, Et3SiH, TMSOTf, CH2Cl2, −78 to −40 °C, 2 h, then, NaH, BnBr, DMF, then, DDQ, H2O, 73% (one pot); (i) benzaldehyde, Et3SiH, TMSOTf, −78 °C, 1.5 h, then, BF3·Et2O, MeCN, −78 to −20 °C, 30 min, then, Bz2O, Et3N, 93% (one pot). Bz, benzoyl; Bz2O, benzoic anhydride; Cu(OTf)2, copper(II) trifluoromethanesulfonate; DDQ, 2,3-dichloro-5,6-dicyano-1,4-benzoquinone; DMAP, 4-(N,N-dimethylamino)pyridine; DMF, N,N-dimethylformamide; Ph, phenyl; PTSA, p-toluenesulfonic acid; TBDPS, tert-butyldiphenylsilyl; THF, tetrahydrofuran; TMS, trimethylsilyl, TMSOTf, trimethylsilyl trifluoromethanesulfonate.