Fig. 1.
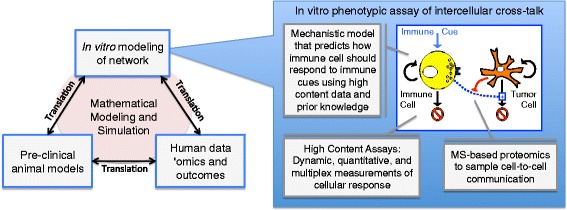
A summary of a quantitative and systems pharmacology approach to identify targets and to improve confidence in the selected targets. An integrated approach for cancer immunology combines in vitro modeling of cellular networks, pre-clinical mouse models of immune-mediated control of tumor growth, and human ’omics and clinical outcomes data from relevant patient populations. Mathematical modeling and simulation aids translational studies by representing the knowledge associated with the relevant biology and by testing this knowledge against data. The box on the right illustrates an example of in vitro modeling of an intercellular network, which is an in vitro phenotypic assay of intercellular cross-talk. The assay incorporates three quantitative and systems pharmacology aspects. First, the assay includes dynamic, quantitative, and multiplex measurements of cellular response. Second, this high content data is interpreted using a mechanistic mathematical model that incorporates prior knowledge about the relevant cell signaling pathways to predict how the immune cell should respond to immune cues, like Interleukin-12. Finally, a mass spectrometry (MS)-based proteomics workflow can be incorporated to characterize secreted cues that influence cell-to-cell communication. The targets that emerge from this integrated process and their associated “wet” and “dry” model systems can provide the rationale for screening for drug candidates using high throughput methods
