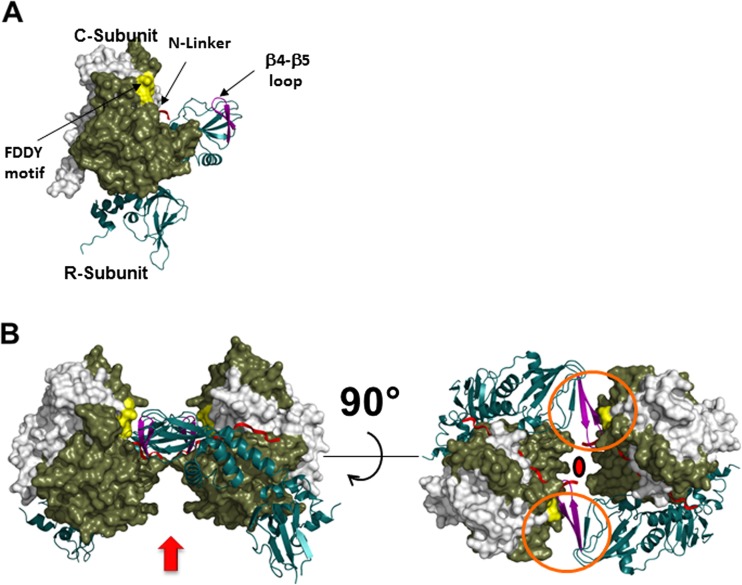
Fig. 4.
Structure of the R2C2 RIIβ holoenzyme. a Structure of the RIIβ:C heterodimer. The RIIβ heterodimer is shown as a ribbon, and the C-subunit is shown as a space-filling model with the N-terminal residues (white residues 14–121, tan C-terminal residues 12–350). Two motifs and the N-linker of the RIIβ-subunit (red) are exposed to solvent in the R heterodimers, but have important roles in the assembly of the holoenzyme. The β4–β5 loop (purple) in the RIIβ holoenzyme is located within the CNB-A domain of the R-subunits. The Phe-Asp-Asp-Tyr (FDDY) motif (yellow) resides in the C-tail of the C-subunit and is an integral part of the ATP-binding site. b Quaternary structure of the R2C2 RIIβ holoenzyme. Arrow Possible position of the D/D domain, red linkers. Rotation allows one to appreciate the twofold symmetry that is found in the holoenzyme and also how the two motifs, the β4–β5 loops and the FDDY motifs (enclosed in orange circles) contribute to the assembly of the holoenyzme. Filled red circle Twofold axis of symmetry