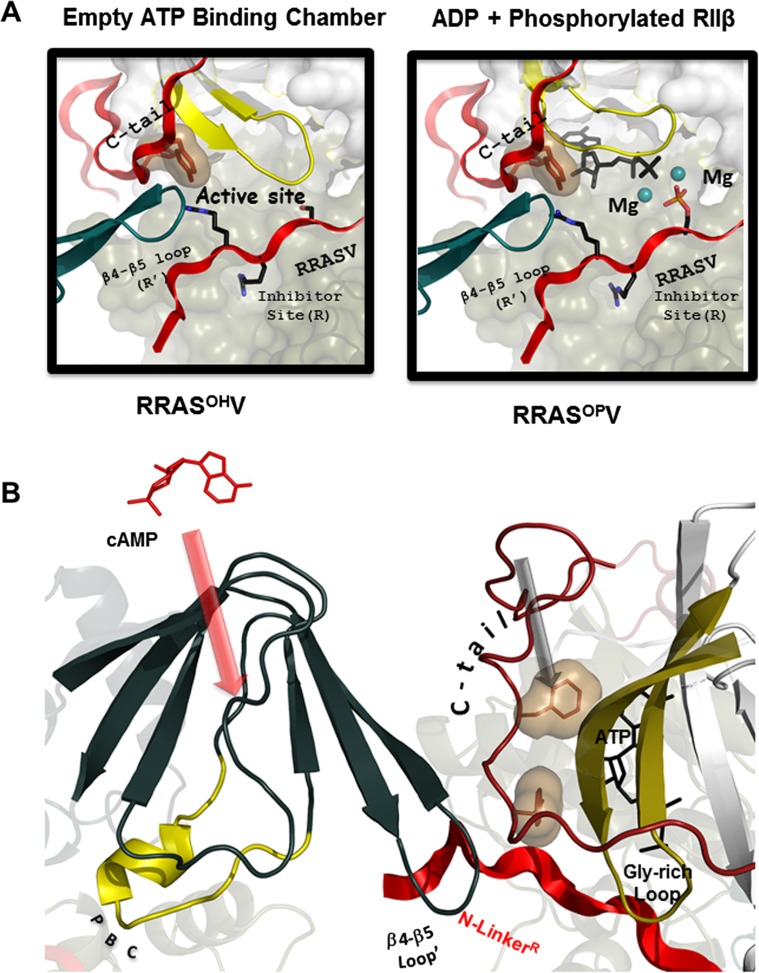
Fig. 6.
Allosteric interactions in the RIIβ holoenzyme allow for single turnover phosphorylation of RIIβ. a Left Active site of the C-subunit in the RIIβ2:C2 tetrameric holoenzyme is fully closed in the absence of ATP, right by diffusing MgATP into the apo-crystals, the reaction products [ADP, (P)-RIIβ and two Mg2+ ions] are trapped. b Binding site for cAMP to CNB-A′ in the R′C′ heterodimer (left arrow) is juxta-positioned against the ATP-binding site in the N-lobe of the C-subunit in the symmetry-related RC dimer. Red arrow Trajectory for binding of cAMP to the PBC