Figure 4.
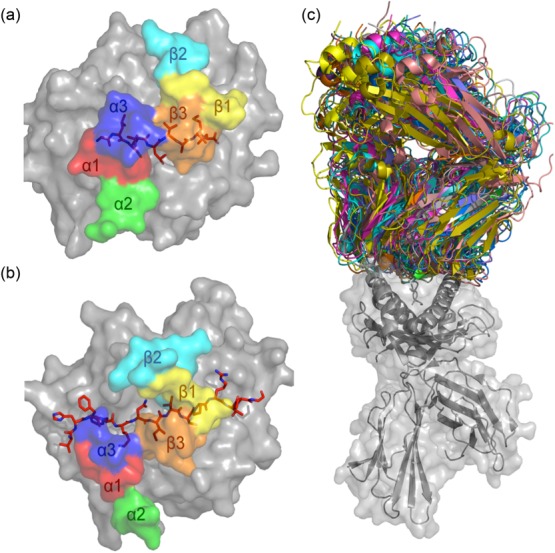
T cell receptor (TCR)–peptide-major histocompatibility complex (pMHC) structures. MHC molecules (in grey) and peptide (in red sticks) are overlaid with the docking footprints of the individual complementarity-determining regions (CDR) loops of the cognate αβTCR. The coloured footprints correspond to the colours of the CDR loops shown in Fig. 2. (a) Structure of MHC-I molecule HLA-A*0201 presenting the immunodominant GLCTLVAML peptide from Epstein–Barr virus (EBV) [Protein Data Bank (PDB): 3O4L] 40. The coloured footprint shows how the CDR loops of the AS01 TCR sit on the pMHC complex. This complex adopts a canonical conformation where the germline-encoded CDR1 and 2 loops contact mainly the MHC and the hypervariable CDR3 loops sit over the peptide. (b) Structure of the MS2-3C8TCR docked on the MHC class II molecule human leucocyte antigen (HLA)-DR4. Here, HLA-DR4 presents a peptide from myelin basic protein (PDB: 3O6F) 41. (c) Overlay of all MHC-I (grey cartoon and surface)-restricted TCRs (multi-coloured) in which co-complex structures have been solved. All complexes were aligned on the MHC-I molecule to demonstrate the flexible nature of TCR–pMHC binding.
