Figure 6.
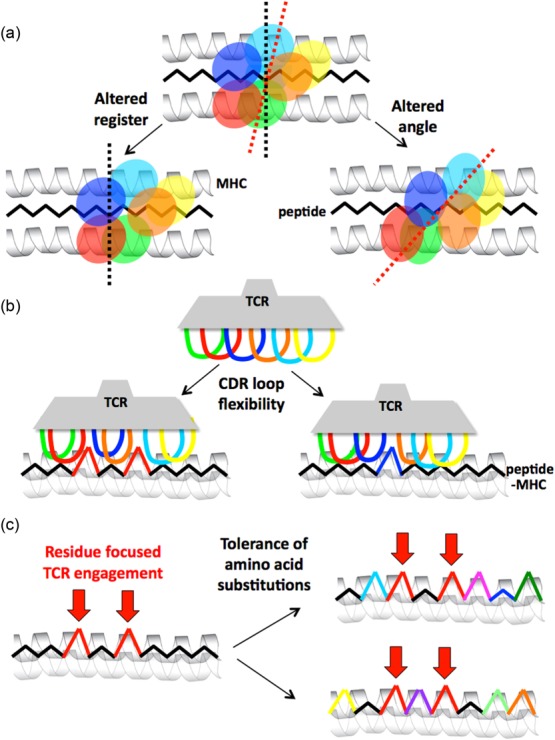
The plasticity of αβ T cell receptor (TCR) binding to peptide-major histocompatibility complex (pMHC). Individual TCRs use multiple mechanisms to bind to pMHC. These effects can increase the number of individual peptides that can be recognized. (a) Macro-level changes enable the TCR to bind pMHC with an altered angle or altered register. The cartoon shows the ‘footprints’ of TCR complementarity-determining region (CDR) loops projected down onto the pMHC. (b) Relatively micro-level flexibility in the CDR loops allows them to accommodate a variety of different shapes. The cartoon shows a side view of a TCR engaging pMHC. (c) The existing database of TCR–pMHC structures indicates that TCRs tend to focus interaction on two to four upward-facing amino acid residues in the antigenic peptide (so-called ‘hotspots’ 63). In this example a TCR might focus on two amino acids in the peptide (shown in red). Such residue-focused interaction then allows the TCR to accommodate multiple amino acid substitutions at other positions in the peptide (indicated by the use of different colours on the right). The three mechanisms described above are not mutually exclusive and represent just some of the possibilities. Many residues can also bind in individual MHC binding pockets. It is now understood that altering a primary MHC anchor can substantially change the way that a peptide might be viewed by incoming T cells 64,65.
