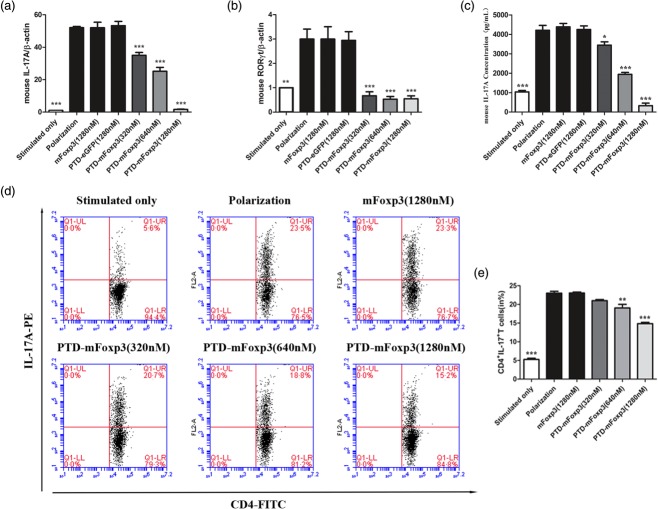
Figure 4.
Protein transduction domain (PTD)-mouse forkhead box protein 3 (mFoxP3) inhibits T helper type 17 (Th17) inducing and interleukin (IL)-17 production. (a) Real-time reverse transcription–polymerase chain reaction (RT–PCR) analysis of transcripts encoding IL-17A in polarized CD4+ T cells. Purified CD4+ T cells were stimulated for 3 days with plate-bound anti-CD3 and soluble anti-CD28 in the presence of anti-interferon (IFN)-γ (10 μg/ml), anti-IL-4 (10 μg/ml), transforming growth factor (TGF)-β (1 ng/ml), IL-1β (10 ng/ml), IL-23 (10 ng/ml) and IL-6 (20 ng/ml), and then treated for another 24 h with 1280 nM PTD-enhanced green fluorescent protein (eGFP) and mFoxP3 and PTD-mFoxP3 (320, 640, 1280 nM) in the presence of neutralizing antibodies and recombinant cytokines but absence of stimulation of anti-CD3 or anti-CD28. RNA was extracted from treated cells, and IL-17A and RORγt mRNA expression was examined by real-time PCR. The data shown were normalized to expression of a reference gene β-actin, and the expression level in stimulated T cells was referred as 1. (b) Real-time RT–PCR analysis of transcripts encoding RORγt in polarized CD4+ T cells. Purified CD4+ T cells were treated as described in (a). (c) Enzyme-linked immunosorbent assay (ELISA) of IL-17A in the supernatants of polarized CD4+ T cells. CD4+ T cells were treated as described in (a), and restimulated with anti-CD3 or anti-CD28 for another 48 h. IL-17A production was assayed by ELISA. (d) Flow cytometry of intracellular cytokine production by total CD4+ T cells under Th17-polarizing condition. IL-17A expression was analysed after 4 h of restimulation with phorbol myristate acetate (PMA)/ionomycin by intracellular cytokine staining. (e) Frequency of CD4+ IL-17+ T cells after PTD fusion proteins treatment. Data are representative of three experiments and are expressed as the mean ± standard deviation. *P < 0·05; **P < 0·01.