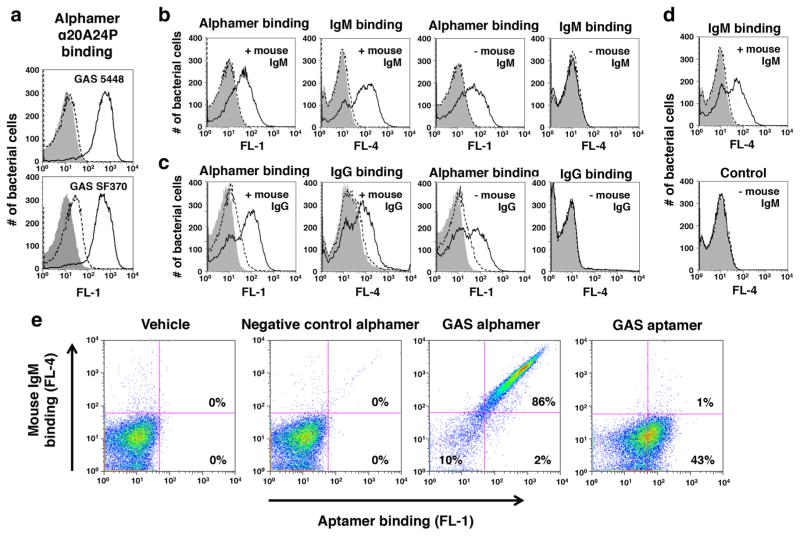
Fig. 5.
Recognition of 5′-α-Gal GAS alphamers on bacteria by anti-α-Gal antibodies. 5′-α-Gal conjugated versions of the GAS aptamers 20A24P (α20A24P) and 20A24P.A3 (α20A24P.A3) as well as negative control RAND-80 (αRAND-80), herein referred to as alphamers, were prepared. a Binding of 5′-α-Gal, 3′-FAM α20A24P (black solid lines) and αRAND-80 (dashed lines) to live GAS 5448 and SF370 cells at an end concentration of 1 μM. The binding of transgenic mouse IgM (b, d) and IgG (c) antibodies to alphamer coated, live GAS 5448 cells was tested by flow cytometry. Bacteria were pre-incubated with 5′-α-Gal, 3′-FAM-labeled GAS alphamer α20A24P (Black solid lines in b, c), non-FAM-labeled 20A24P.A3 (black solid lines in d), 3′-FAM-labeled control alphamer αRAND-80 (dashed lines) or alphamer vehicle (gray solid). The binding of the primary antibodies was detected with secondary Alexa Fluor® 647 anti-mouse IgM (b, d) or anti-mouse IgG (c) antibodies. This allowed for simultaneous visualization of alphamer and mouse antibody binding in the FL-1 and FL-4 channels, respectively. Samples incubated with alphamers but no mouse IgM or IgG (–mouse IgM; –mouse IgG) served as negative controls to rule out unspecific secondary antibody binding to the bacteria. Representative results of several experiments performed are shown in the histogram overlays. e To determine if the α-Gal portion was critical for mouse antibody binding to 5′-FAM-α20A24P-coated GAS 5448, the ability of the alphamer and non-α-Gal-conjugated aptamer 5′-FAM-20A24P at 1 μM endconcentration to redirect mouse IgM antibodies to the bacteria was compared as above. Vehicle and 5′-FAM-αRAND-80 were used as controls. FL-1 (= Aptamer/alphamer binding) vs. FL-4 (= IgM binding) dot blots for one representative experiment of several performed are shown