Abstract
The establishment of the head to tail axis at early stages of development is a fundamental aspect of vertebrate embryogenesis. In mice, experimental embryology, genetics and expression studies have suggested that the visceral endoderm, an extra-embryonic tissue, plays an important role in anteroposterior axial development. Here we show that absence of Wnt3 in the posterior visceral endoderm leads to delayed formation of the primitive streak and that interplay between anterior and posterior visceral endoderm restricts the position of the primitive streak. Embryos lacking Wnt3 in the visceral endoderm, however, appear normal by E9.5. Our results suggest a model for axial development in which multiple signals are required for anteroposterior axial development in mammals.
Introduction
The establishment of the head to tail axis is a fundamental step in generating the basic body plan of vertebrates (Stern et al., 2006). In amniote embryos, like those of birds and mammals, this axis is marked at its caudal end by the primitive streak, a region of epithelial to mesenchymal transition that serves as a conduit for the generation of mesoderm and endoderm during gastrulation (Stern, 2004). In mammals, understanding how the primitive streak forms has special significance because deciphering why the primitive streak forms where it does can explain how the anteroposterior axis is established during embryogenesis (Beddington and Robertson, 1998).
In mice, two components of the early post-implantation embryo, the extra-embryonic ectoderm and the posterior visceral endoderm, have been proposed to induce the formation of the primitive streak (Bachvarova, 1996; Beddington and Robertson, 1999; Conlon and Beddington, 1995). The extra-embryonic ectoderm is a trophectoderm derivative that abuts the epiblast, the tissue that gives rise to the primitive streak. Several lines of evidence suggest that Bmp4 emanating from the extra-embryonic ectoderm signals to the adjacent epiblast to activate primitive streak markers (Arnold and Robertson, 2009; Beddington and Robertson, 1999; Ben-Haim et al., 2006). The induced epiblast then undergoes an epithelial to mesenchymal transition to become the primitive streak, allowing gastrulation and the subsequent generation of mesoderm and endoderm.
A less studied potential signaling center is the posterior visceral endoderm, an extra-embryonic component derived from primitive endoderm located adjacent to the region of epiblast that becomes the primitive streak. Tissue recombination experiments have shown that the posterior visceral endoderm has the capacity to re-specify anterior ectoderm into a posterior mesodermal fate and that this reprograming is effected by a diffusible signal (Belaoussoff et al., 1998). A candidate molecule is Wnt3; Wnt3 knockout embryos lack a primitive streak and fail to gastrulate (Liu et al., 1999). In addition, Wnt3 expression is first observed in the posterior visceral endoderm of embryos dissected at embryonic day 5.5 and expands to the adjacent epiblast tissue a few hours later (Rivera-Perez and Magnuson, 2005). In support of the hypothesis that Wnt3 derived from visceral endoderm has an inductive role in primitive streak formation, embryos in which Wnt3 was conditionally inactivated in the epiblast were able to establish the primitive streak and initiate gastrulation although they failed to thrive soon afterwards (Tortelote et al., 2013). Intriguingly, in Wnt3-epiblast mutant embryos, Wnt3 mRNA is present transiently in the posterior visceral endoderm suggesting that Wnt3 signaling from the posterior visceral endoderm is responsible for the initiation of gastrulation (Tortelote et al., 2013).
In order to determine if Wnt3 function in the posterior visceral endoderm is required for the formation of the primitive streak, we inactivated Wnt3 in the posterior visceral endoderm using Transthyretin-Cre mice. Our results show that embryos lacking Wnt3 solely in the visceral endoderm specify the primitive streak later than control littermates. This effect is exacerbated if a copy of Wnt3 is additionally inactivated in the epiblast. Despite these defects, Wnt3 visceral endoderm mutant embryos are able to gastrulate and develop normally. From these results, we conclude that Wnt3 function in the posterior visceral endoderm regulates the formation of the primitive streak and gastrulation but its function is dispensable for embryo development. Our results also indicate that the anterior visceral endoderm plays a role in preventing the expansion of Wnt3 signaling to the anterior side of the embryo restricting the formation of the primitive streak to the opposite side of the epiblast. We propose a model in which a series of coordinated events involving the visceral endoderm, extra-embryonic ectoderm and epiblast control the development of the primitive streak in mammals.
Materials and Methods
Embryo staging and mice
Embryos were staged based on morphological landmarks as previously described (Downs and Davies, 1993; Rivera-Perez et al., 2010) or described in terms of dissection time. Noon of the day that a mating plug was observed was considered embryonic day 0.5 (E0.5) of gestation. TtrCre mice were previously described (Kwon and Hadjantonakis, 2009). Wnt3c mice were obtained from Dr. Jeff Barrow (Barrow et al., 2003). Cripto mutant mice (CriptolacZ) were provided by Dr. Michael Shen (Ding et al., 1998). HexGFP mice were provided by Dr. Tristan Rodriguez (Rodriguez et al., 2001). All Wnt3 mutant alleles, TtrCre, CriptolacZ and HexGFP mice were maintained on a CD-1 outbred genetic background.
Generation of Wnt3lacZ knock-in mice
To target the Wnt3 locus, we designed a targeting vector containing a 6 kb NotI-BamHI genomic DNA fragment (129S6/SvEvBrd) that included exons 3 and 4. A lacZ cassette and a floxed PGKneobpA cassette were inserted into the ClaI site in exon 4. The IacZ cassette contained an internal ribosomal entry site (IRES) and an SV40 polyA signal sequence. An HSV-tkpA cassette was added to the 5′-homologous arm. Gene targeting was conducted in AB1 ES cells as previously described (Mishina et al., 1995). A total of 192 G418; FIAU double-resistant ES cell colonies were screened for homologous recombination by Southern blotting using a Wnt3 3′ UTR probe (Liu et al., 1999). Twenty-seven lines were positive in the initial screening, and two lines gave rise to germ-line chimeras. We obtained Wnt3lacZneo heterozygous mice after crossing chimeras to C57BL/6 mice. Wnt3lacZneo heterozygous mice were crossed to Sox2Cre mice (Hayashi et al., 2002) to remove the neo cassette and generate Wnt3lacZ heterozygous mice. Heterozygous mice for both alleles were normal and fertile. To determine whether the Wnt3lacZneo and Wnt3lacZ alleles are null alleles, we generated Wnt3lacZneo/lacZneo and Wnt3lacZ/lacZ homozygous embryos or crossed Wnt3lacZneo and Wnt3lacZ heterozygous mice with mice carrying the Wnt3Δ3,4 null allele (Barrow et al., 2003) to generate Wnt3lacZneo/Δ3,4 and Wnt3lacZ/Δ3,4 mutant embryos. All types of mutants phenocopy the previously reported phenotype of Wnt3 null embryos (Barrow et al., 2007; Liu et al., 1999), that is, they lack a primitive streak, fail to elongate the anteroposterior axis and are resorbed by ~E9.5 indicating that they are null alleles. The Wnt3lacZneo strain has been registered with the Mouse Genome Informatics international database under the identifier MGI:5439832 (Wnt3tm1Bhr).
Germ layer isolation
To separate the epiblast from the visceral endoderm, we followed the germ layer separation protocol described previously (Nagy, 2003). Briefly, embryos were cut just below the epiblast –extra-embryonic ectoderm boundary, incubated in a solution containing a mixture of pancreatin and trypsin and pipetted up and down using a pulled Pasteur pipette. Germ layer separation was performed retrospectively after wholemount in situ hybridization was conducted.
Whole-mount in situ hybridization
Whole-mount in situ hybridization was performed as previously described (Rivera-Perez and Magnuson, 2005) using the following probes: Wnt3 (full length cDNA, 2,084 bp). Brachyury (full length cDNA, 1,784 bp) (Herrmann, 1991). All riboprobes were prepared using a digoxigenin RNA labeling kit (Roche Cat. No. 1175025).
β-Galactosidase staining protocol
We performed β-galactosidase assays as described by Sundararajan and co-workers (Sundararajan et al., 2012). After β-galactosidase staining, embryos were rinsed in wash solution re-fixed in 4% paraformaldehyde at room temperature for 20 min., cleared in glycerol and imaged.
Genotyping
The genotype of the embryos was determined retrospectively after wholemount in situ hybridization or after β-galactosidase assays. Each litter was imaged before genotyping to allow unique identification of each embryo. Embryos were placed in 15–20 μl of PCR lysis buffer (50 mM KCl, 10 mM Tris-HCl pH8.3, 2.5 mM MgCl2, 0.1 mg/ml gelatin, 0.45% IGEPAL (Sigma, Cat. No. 18896) and 0.45% Tween 20) containing 100 mg/ml Proteinase K and incubated overnight at 56°C. After lysis, the proteinase K was inactivated at 95°C for 5 min and 1 μl of the lysate was used for the PCR reaction. Mice were genotyped at postnatal day 10 using a 2 mm tail tip piece.
PCR reactions were carried out using the following oligonucleotides: Wnt3 wild-type allele, ptmw1 GAC TTC CTC AAG GAC AAG TAC G and ptmw2 GAA GAC GCA ATG GCA TTT CTC (309 bp); Wild-type and Wnt3c alleles, Wnt3F3 5′ TGG CTT CAG CAT CTG TTA CCT TC 3′ and Wnt3R6 5′ AAG ATC CCC ATA CTG CCA TCA C 3′ (389 bp and 546 bp, respectively); Wnt3Δ3,4 allele, Wnt3F10 5′ GGG AGC CGC CCG TTG CTA 3′ and Wnt3R6 5′ AAG ATC CCC ATA CTG CCA TCA C 3′ (388 bp); Wnt3lacZ allele, LacZF 5′TGG CGT TAC CCA ACT TAA TCG 3′ and LacZR 5′ATG TGA GCG AGT AAC AAC CCG 3′ (324 bp); Wnt3lacZneo allele, NeoF2 TGG CTA CCC GTG ATA TTG CTG and ptmw2 GAA GAC GCA ATG GCA TTT CTC (~500 bp); TtrCre transgene, qCreF1 5′ GAA CCT CAT GGA CAT GTT CAG G 3′ and qCreR1 5′ AGT GCG TTC GAA CGC TAG AGC CTG T 3′ (320 bp); Cripto wild type allele, Cripto5′UTRf CCT CCG AAG TCC TCA ATC AC and CriptoR3 TCC GAA GTG GCT ATC TCC AG (333 bp); CriptolacZ Cripto5′UTRf CCT CCG AAG TCC TCA ATC AC and CriptolacZ GAT TAA GTT GGG TAA CGC CAG (~300 bp).
Statistical analysis
We used the Student t-test to determine if there was significant difference between the extent of T expression relative to epiblast length among Wnt3-VE mutants (Wnt3c/c;TtrCre/0) and control (Wnt3c/c) littermates.
Results
Visceral endoderm Wnt3 regulates its own expression in the epiblast
Previous experiments have shown that expression of Wnt3 in the posterior visceral endoderm appears at ~E5.5 and then in the adjacent epiblast six hours later at ~E5.75 (Rivera-Perez and Magnuson, 2005). In addition, embryos in which Wnt3 was inactivated in the epiblast but not in the visceral endoderm were able to form a primitive streak, suggesting a signaling role for visceral endoderm-derived Wnt3 in this process (Tortelote et al., 2013). To explore the ability of visceral endoderm-derived Wnt3 to signal to the epiblast we took advantage of Wnt3lacZ mice. Wnt3lacZ mice have the lacZ gene inserted in exon 4 of the Wnt3 locus (Fig. 1A), making it possible to monitor the activity of the Wnt3 locus using β-galactosidase (β-gal) assays. We reasoned that if Wnt3 is first activated in the visceral endoderm and it is required for its own expression in the epiblast, Wnt3lacZ/lacZ mutant embryos should show β-galactosidase activity in the visceral endoderm but not in the epiblast.
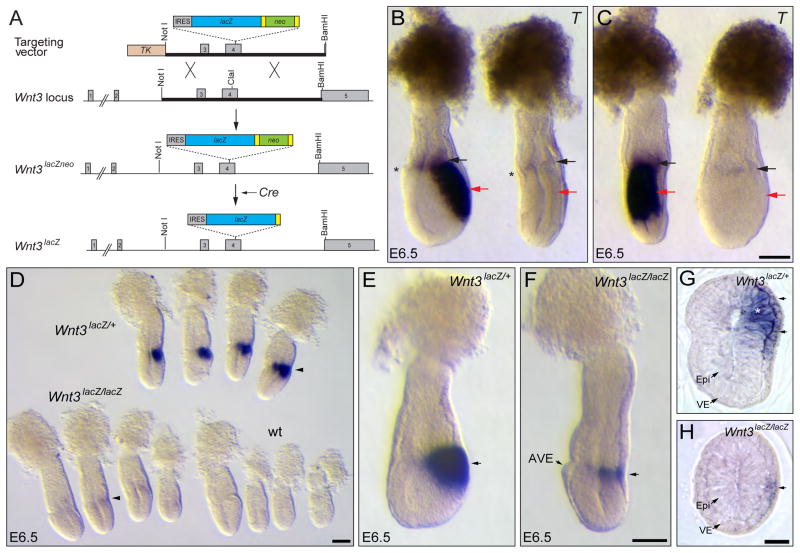
Figure 1. Visceral endoderm Wnt3 regulates its own expression in the epiblast.
A. Schematic representation of the strategy for the generation of Wnt3lacZNeo and Wnt3lacZ alleles. A replacement vector carrying an IRES-lacZ-loxP-Neo-loxP cassette inserted into the ClaI site of exon 4 was used to generate Wnt3lacZNeo/+ mice. Heterozygous Wnt3lacZNeo/+ mice were crossed to Sox2Cre mice to remove the Neo cassette and generate Wnt3lacZ/+ animals. B, C. Lateral (B) and posterior (C) view of the same control and Wnt3lacZ/lacZ mutant embryos hybridized with T The Wnt3lacZ/lacZ mutant embryo lacks expression of T in the epiblast (red arrows). Faint T expression is observed in the extra-embryonic ectoderm of the mutant embryo (black arrows). The mutant embryo shows the characteristic flattened anteroposterior morphology of Wnt3 null embryos, indicating that Wnt3lacZ is a null allele. The anterior visceral endoderm (AVE), indicating the anterior side of the embryo, is marked with an asterisk. D. Analysis of β-galactosidase activity in embryos derived from Wnt3lacZ/+ heterozygous crosses at E6.5. Strong staining is observed in the posterior region of heterozygous (Wnt3lacZ/+) embryos, while only weak staining is observed in mutant (Wnt3lacZ/lacZ) embryos (arrowheads) and no staining in wild-type (wt) embryos. E, F. Wholemount β-galactosidase activity assay of heterozygous (E) or homozygous (F) embryos for Wnt3lacZ alleles dissected at a slightly older stage than those shown in D. In the homozygous embryo, β-galactosidase activity is restricted to a narrow band on the posterior side (arrow) opposite to the AVE. G, H. Cross sections of heterozygous (G) and mutant (H) Wnt3lacZ embryos assayed for β-galactosidase activity. Heterozygous embryos show β-galactosidase activity in the epiblast (asterisk) and visceral endoderm (arrows), whereas mutant embryos exhibit staining only in the visceral endoderm (arrow). IRES, internal ribosomal entry site. TK, Thymidine Kinase cassette; Yellow rectangles represent loxP sites. Epi, epiblast; VE, visceral endoderm. Panels B and C, E and F and G and H are shown at the same scale. Scale bars, 100 μm in C, D, F and H.
We demonstrated that the Wnt3lacZ allele is a null allele of Wnt3 by showing absence of Brachyury (T) expression in the epiblast of Wnt3lacZ/lacZ mutant embryos and by failure to undergo elongation of the anteroposterior axis (Fig. 1B, C). This phenotype was reiterated in mutants obtained from crosses between Wnt3lacZ mice and mice carrying the Wnt3Δ3,4 null allele (Barrow et al., 2007) (not shown).
Analysis of E6.5 litters obtained from heterozygous Wnt3lacZ crosses revealed embryos with strong, weak or no β-gal activity (Fig. 1D). Retrospective embryo genotyping showed that Wnt3lacZ heterozygous embryos (n=7) had strong staining at the epiblast/extra-embryonic ectoderm boundary on one side of the embryo (Fig. 1D, E), mimicking the pattern of Wnt3 expression (Rivera-Perez and Magnuson, 2005; Sundararajan et al., 2012). Wnt3lacZ homozygous embryos (n=7), on the other hand, showed a weak area of staining restricted to half the circumference of the proximal posterior region of the embryo and extended distally only about one fourth of the length of the epiblast region (Fig. 1D, F). As expected, no staining was observed in wild-type embryos (Fig. 1D). Histological analysis revealed that heterozygous embryos exhibited strong β-gal activity in both the posterior visceral endoderm and adjacent epiblast (Fig. 1G), while staining in mutant embryos was restricted to the visceral endoderm layer (Fig. 1H).
The observed β-gal activity in both the epiblast and visceral endoderm of heterozygous embryos but only in the posterior visceral endoderm of mutant embryos suggests that Wnt3 signaling derived from the posterior visceral endoderm is required for its own expression in the adjacent epiblast at E6.5.
Wnt3 in the visceral endoderm is dispensable for primitive streak formation
To determine the requirement for visceral endoderm-derived Wnt3 in the establishment of the primitive streak, we inactivated Wnt3 in the visceral endoderm using mice carrying a conditional allele of Wnt3 (Wnt3c) (Barrow et al., 2003) and TtrCre transgenic mice (Kwon and Hadjantonakis, 2009). The Wnt3c allele has loxP sites flanking exons 3 and 4 and generates the Wnt3Δ3,4 null allele after Cre-mediated recombination (Fig. 2A) (Barrow et al., 2003). TtrCre transgenic mice express Cre recombinase under the control of the Transthyretin (Ttr) promoter in the visceral endoderm of early post-implantation embryos at ~E5.0 (Kwon and Hadjantonakis, 2009).
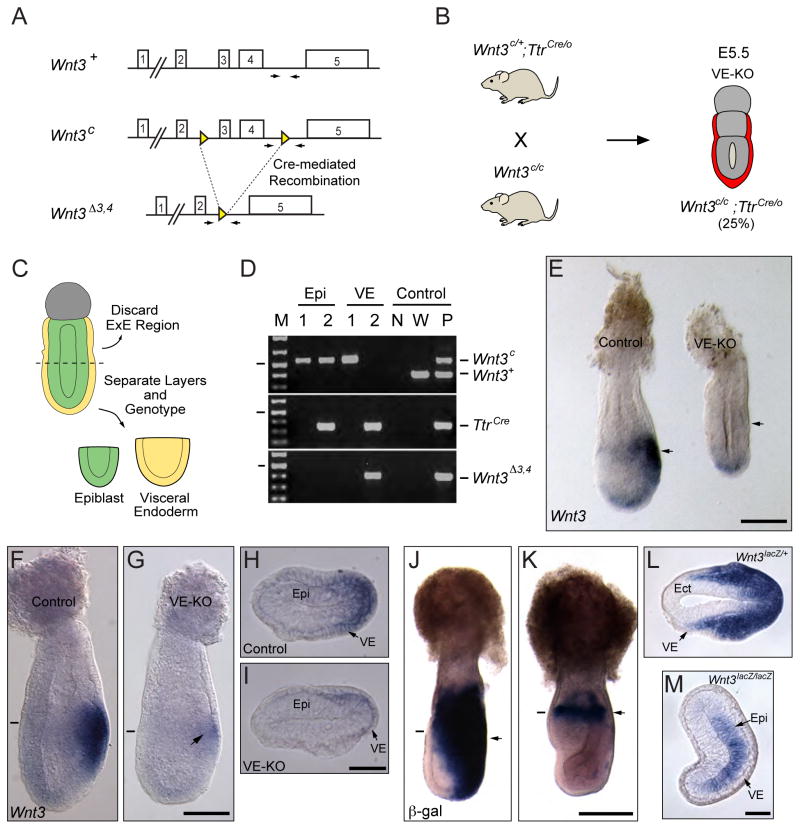
Fig. 2. Wnt3 function in the visceral endoderm is dispensable for Wnt3 expression in the epiblast.
A. Schematic representation of Wnt3 wild-type (Wnt3+), floxed (Wnt3c) and null (Wnt3Δ3,4) alleles. Arrows indicate the position of the oligos used for genotyping. B. Mating strategy for generating embryos with conditional ablation of Wnt3 in the visceral endoderm. Wnt3-VE null embryos are mutant for Wnt3 in the visceral endoderm (red) but retain two functional alleles of Wnt3 (Wnt3c) in the rest of the conceptus. C. Schematic representation of the strategy for isolating the epiblast and visceral endoderm layers from control and Wnt3-VE mutant embryos. D. PCR-genotyping of the epiblast and visceral endoderm from control and Wnt3-VE embryos. Genotyping for Cre indicates that only sample 2 is positive for the TtrCre transgene (320 bp). Therefore, sample 1 corresponds to a Wnt3c/c and sample 2 to a Wnt3c/c;TtrCre/0 embryo. Both samples retain the non-recombined Wnt3c allele (546 bp) but not the wild-type (381 bp) or recombined Wnt3Δ3,4 (388 bp) alleles in the epiblast. The presence of the Wnt3Δ3,4 allele and the absence of the Wnt3c allele in the visceral endoderm of sample 2 indicates that the TtrCre transgene induced a visceral endoderm-specific recombination of the Wnt3c allele in this tissue. The oligos used to amplify the Wnt3c allele also amplify the Wnt3+ allele, as seen in wild type (W) and positive (P) control samples. M, 1kb plus DNA ladder. The lines on the left indicate the 500 bp band. N, no template control. E. E6.25 control and Wnt3-VE mutant (VE-KO) embryos hybridized with a Wnt3 probe. The VE-KO embryo lacks Wnt3 expression whereas the control embryo exhibits the Wnt3 expression in the posterior region (arrows). Background staining is evident in the distal region of both embryos. F, G. E6.5 control and Wnt3-VE mutant embryos hybridized with Wnt3 probe. The control embryo exhibits expression of Wnt3 in the posterior epiblast and visceral endoderm. The VE-KO embryo has weak Wnt3 expression in the epiblast (arrow). H, I. Histological sections from control (H) and VE-KO mutant (I) embryos shown in F and G, respectively. The approximate position of the sections is indicated by a small line. J, K. E7.25 heterozygous (Wnt3lacZ/+) or homozygous (Wnt3lacZ/lacZ) embryos assayed for β-galactosidase activity. The heterozygous embryo exhibits β-galactosidase staining in the primitive streak and its mesendoderm descendants while the homozygous embryo has β-galactosidase activity restricted to a small area of the epiblast (arrows). L, M. Histological sections of the embryos shown in Panels J and K. The approximate location of the sections is indicated by a small line. Panels F and G, H and I, J and K and L and M are shown at the same scale. Scale bars, 100 μm in E, G, I and M; 200 μm in K.
To generate Wnt3 visceral endoderm knockout (VE-KO) embryos we initially crossed males heterozygous for the conditional Wnt3c allele and hemizygous for the TtrCre transgene (Wnt3c/+;TtrCre/0) with females homozygous for the Wnt3c allele (Wnt3c/c). However, we observed embryos that carried a recombined Wnt3 allele (Wnt3Δ3,4) even in the absence of the TtrCre transgene. This phenomenon was confirmed by crossing Wnt3c/+;TtrCre/0 males with CD-1 wild-type females and genotyping of E9.5 embryos. The genotype was determined using only the head of the embryos to avoid contamination with visceral endoderm derivatives from the visceral yolk sac or the gut (Kwon et al., 2008). Approximately 50% (50/85) of embryos obtained from crosses of Wnt3c/+;TtrCre/0 males and wild-type females were heterozygous for the Wnt3Δ3,4 allele while none of them carried the Wnt3c allele indicating complete recombination in the paternal germline. We also tested whether recombination of the conditional allele occurred in the female germline. Crosses between Wnt3c/+;TtrCre/0 females with wild-type males revealed recombination in a subset of oocytes. From a total of 37 E9.5 embryos analyzed from these crosses, we observed that 18 embryos had inherited the Wnt3c allele and 4 of them had undergone recombination to generate the Wnt3Δ3,4 allele, corresponding to a frequency of 22% maternal germline recombination. Despite these drawbacks we were able to generate Wnt3-VE mutant embryos from Wnt3c/+;TtrCre/0 female by Wnt3c/c male crosses (Fig. 2B) that were unequivocally identified by genotyping the epiblast and visceral endoderm components of the conceptus (Fig. 2C, D).
Embryos were first hybridized with a Wnt3 probe to confirm ablation of Wnt3 in the visceral endoderm. Analysis of embryos at E6.25 revealed absence of Wnt3 expression in both the epiblast and the visceral endoderm in mutant embryos (n=3) (Fig. 2E). At E6.5, however, weak Wnt3 expression became evident in the epiblast of Wnt3-VE mutant embryos (n=5) (Fig. 2F–I). These results show that Wnt3 expression was abolished in the visceral endoderm of Wnt3-VE mutant embryos. At the same time, the presence of Wnt3 transcripts in the epiblast of Wnt3-VE mutants suggests that Wnt3 activity emanating from the posterior visceral endoderm is not solely responsible for inducing Wnt3 expression in the epiblast. We confirmed these results by analyzing the expression of the Wnt3lacZ transgene in Wnt3lacZ/lacZ mutant embryos at E7.5 (n=19). In these embryos, we were able to detect weak β-galactosidase activity in the epiblast (Fig. 2J–M). This data suggests that Wnt3 and other factors control the activity of Wnt3 in the epiblast.
Inactivation of Wnt3 in the visceral endoderm leads to delayed formation of the primitive streak
To further characterize the phenotype of Wnt3-VE mutant embryos, we performed wholemount in situ hybridization using a T probe. T marks the primitive streak and its precursors in the epiblast (Rivera-Perez and Magnuson, 2005; Wilkinson et al., 1990). We found that Wnt3-VE mutant embryos exhibited T expression at E6.5, however, this expression tended to be weak and occupied a smaller area that in control littermates (Fig. 3A, B). To quantify the level of T expression in Wnt3-VE mutant embryos, we measured the extent of T expression in the posterior epiblast relative to epiblast length (T/Epi ratio) and compared it to control littermates using a t-test (Fig. 3C, Supp. Fig. 1). We found that Wnt3-VE null embryos (Wnt3c/c;TtrCre/0, n=10) had a smaller area of T expression compared to controls (Wnt3c/c, n=18), indicative of delayed development (p<0.001). Analysis of embryos at E.9.5 showed that Wnt3-VE null embryos (Wnt3c/c;TtrCre/0) were indistinguishable from control littermates (not shown) suggesting that Wnt3-VE mutant embryos reach normal development soon after gastrulation. From these results, we conclude that Wnt3 function in the visceral endoderm regulates the timing of primitive streak formation but it is dispensable for further embryonic development.
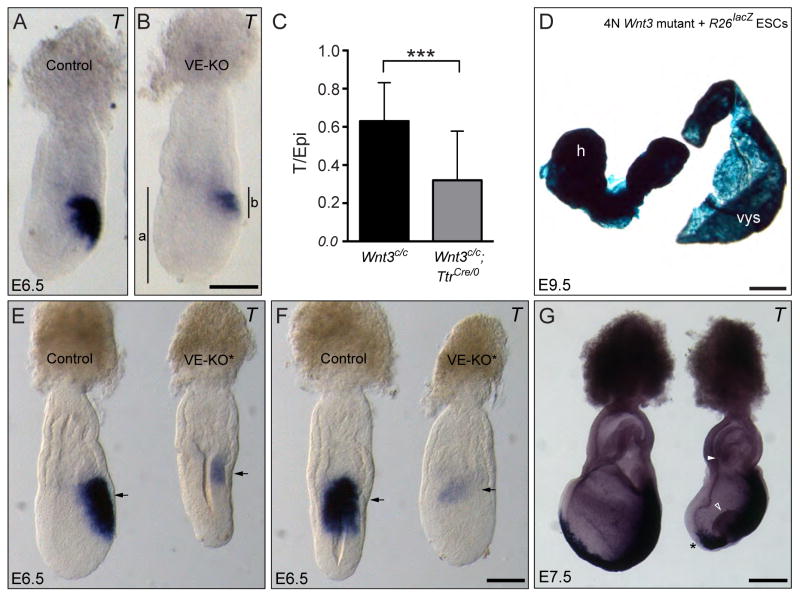
Fig. 3. Inactivation of Wnt3 in the visceral endoderm leads to delayed formation of the primitive streak.
A, B. Lateral views of control (A) and Wnt3 VE-null (VE-KO) (B) embryos hybridized with T. The vertical lines in B, indicate the length of the epiblast (a) and primitive streak (b) as indicated by T expression. C. Analysis of the extent of primitive streak development between control (Wnt3c/c) and VE-KO (Wnt3c/c;TtrCre/0) embryos. A comparison of the ratio of the extent of T expression relative to the proximodistal length of the epiblast (as indicated in B) reveals significant delay in the development of the primitive streak in VE-KO embryos relative to controls (p<0.001). D. Chimera derived from a tetraploid Wnt3 null (Wnt3lacZNeo/Δ3,4) blastocyst injected with R26lacZ ES cells. Embryo is composed solely of ES cells and has undergone gastrulation, indicating that Wnt3 function in the visceral endoderm is dispensable for gastrulation. The yolk sac appears blue due to the presence of β-gal positive extra-embryonic mesoderm cells derived from the R26lacZ ES cells. h, head; vys, visceral yolk sac. E – G. Lateral (E) and posterior (F) views of control (Wnt3c/Δ3,4) and Wnt3-VE mutant embryos heterozygous for Wnt3 in the epiblast (VE-KO*; Wnt3c/Δ3,4;TtrCre/0) hybridized with T at E6.5 (E and F) and E7.5 (G). The Wnt3c/Δ3,4;TtrCre/0 embryo fails to elongate the anteroposterior axis at E6.5 and has abnormal gastrulation at E7.5 as shown by the presence of a bulge of cells in the primitive streak (arrow), failure to close the proamniotic canal (arrowhead) and absence of T staining in axial mesoderm (asterisk). Panels A and B and E and F are shown at the same scale. Scale bars, 100 μm in B and F, 500 μm in D, and 200 μm in G.
To independently confirm that Wnt3 function in the visceral endoderm is not essential for primitive streak formation, we used tetraploid complementation chimeras. We generated tetraploid embryos from crosses between mice carrying two different Wnt3 null alleles (Wnt3lacZneo/+ X Wnt3Δ3,4/+). In this way, mutant chimeras are unequivocally identified by the presence of two mutant Wnt3 alleles in the yolk sac. Tetraploid blastocysts were injected with ES cells carrying the R26lacZ allele (Tremblay et al., 2000), chimeras were recovered at E9.5 and assayed for β-galactosidase activity. We obtained six chimeras of which two of them were derived from mutant Wnt3lacZneo/Δ3,4 tetraploid blastocysts. These embryos were able to undergo gastrulation and reached the initial stages of organogenesis (Fig. 3D), supporting our genetic results.
Reduced Wnt3 activity in the epiblast of Wnt3-VE null mutants leads to gastrulation defects
Recombination of the conditional Wnt3 allele in the maternal germline sometimes led to the generation of Wnt3-VE null embryos that carried one Wnt3 mutant allele in the epiblast. This resulted in embryos lacking Wnt3 in the visceral endoderm (Wnt3Δ3,4/Δ3,4) with a reduced dose of Wnt3 function in the epiblast (Wnt3Δ3,4/c). We noticed that these embryos exhibited a more severe phenotype than Wnt3-VE null mutants containing two Wnt3c alleles in the epiblast (Wnt3c/c). To further explore this phenotype, we crossed Wnt3Δ3,4/+;TtrCre males and Wnt3c/c females to generate Wnt3Δ3,4/c;TtrCre embryos. At E6.5 (n=5), these embryos showed delayed development with failure to elongate the epiblast and incipient T expression (Fig. 3E, F). Morphological defects were more pronounced at E7.5 (n=6). At this stage, embryos showed a bulging in the primitive streak, failure to close the proamniotic canal and failure to generate axial mesoderm (Fig. 3G). This phenotype is similar to the phenotype observed in Wnt3 epiblast knockout embryos (Tortelote et al., 2013). These results indicate that a combination of Wnt3 visceral endoderm signaling and proper Wnt3 dosage in the epiblast is necessary for gastrulation.
The anterior visceral endoderm restricts expression of Wnt3 to the posterior side of the embryo
Analysis of Wnt3 expression relative to the AVE marked by Hex-GFP revealed that expression of Wnt3 is complementary to the AVE (Fig. 4A–C). This observation led us to postulate that the AVE prevents Wnt3 activity in the anterior side of the embryo. To address this question, we took advantage of Cripto mutant embryos. In Cripto mutants, the AVE fails to localize to the anterior side of the epiblast and remains at the distal tip of the conceptus (Ding et al., 1998). This misallocation of the AVE leads to radialization of the primitive streak in the proximal epiblast as indicated by the expression of T and other markers. Since T expression in the epiblast depends on Wnt3 (Barrow et al., 2007; Liu et al., 1999; Tortelote et al., 2013), we wondered if Wnt3 expression was radialized in Cripto mutant embryos.
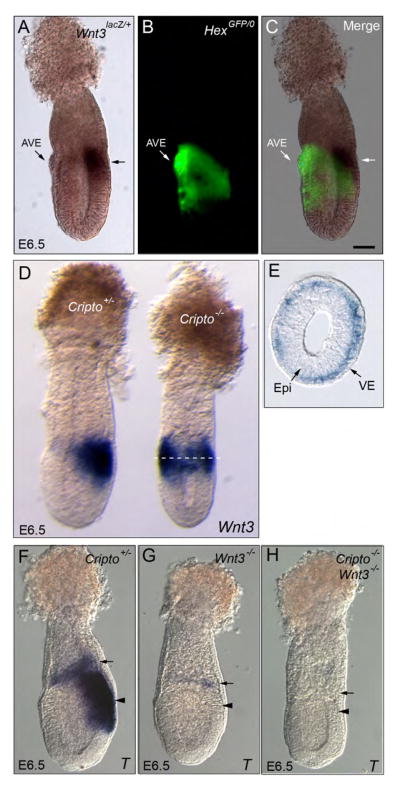
Fig. 4. The AVE restricts Wnt3 expression to the posterior side of the embryo.
A–C. E6.5 embryos double heterozygous for HexGFP and Wnt3lacZ assayed for β-galactosidase activity. Wnt3 expression (arrows) is complementary to the AVE (marked by HexGFP). D. Heterozygous and Cripto mutant embryos dissected at E6.5 and hybridized with a Wnt3 probe. In the Cripto mutant embryo, Wnt3 expression is radialized in the proximal epiblast region. E. Transverse section of Cripto mutant embryo shown in D. The approximate location of the section is marked by a dotted line. Cripto expression is radialized but restricted to the visceral endoderm layer. F–H. Cripto Heterozygous (F), Wnt3 mutant (G) and double Wnt3/Cripto mutant (H) embryos dissected at E6.5 and hybridized with T. T is expressed in the extra-embryonic ectoderm (arrow) and epiblast (arrowhead) of heterozygous embryos and weakly in the extra-embryonic ectoderm of Wnt3 mutants. The double mutant embryo completely lacks T expression showing that in Cripto mutant embryos, the radialization of T in the epiblast depends on radialized Wnt3 activity in the visceral endoderm.
Analysis of Wnt3 expression in Cripto null embryos (n=11) revealed that Wnt3 expression was indeed radialized in mutant embryos forming a ring of expression around the proximal epiblast region (Fig. 4D). Surprisingly, histological analysis showed that Wnt3 expression was restricted to the visceral endoderm layer and was absent from the epiblast (Fig. 4E). These results indicate that the AVE prevents the expansion of Wnt3 signaling to the anterior side of the embryo and that Wnt3 expression is downstream of Cripto in the epiblast.
To determine if the ectopic expression of Wnt3 in the visceral endoderm of Cripto mutants was responsible for the expression of T in the anterior epiblast, we removed Wnt3 activity from Cripto null embryos by generating Cripto/Wnt3 double mutant embryos. We generated 54 embryos from double heterozygous (Wnt3lacZ/+;Cripto+/−) crosses and obtained 5 Cripto/Wnt3 double mutant embryos (Fig. 4F–H). We did not detect T expression in these double mutant embryos (Fig. 4H). Therefore, the radialized expression of Wnt3 in the proximal visceral endoderm of Cripto mutants leads to ectopic activation of T in the anterior epiblast. These results suggest that Wnt3 is sufficient to induce primitive streak markers in a non-cell autonomous manner at an ectopic location.
Discussion
Previous studies showed that a diffusible signal derived from the visceral endoderm was able to reprogram anterior epiblast to a posterior mesodermal fate to produce hematopoietic tissues (Belaoussoff et al., 1998). Recent evidence further implicated the visceral endoderm as the source of an inductive signal that directs the formation of mesoderm during gastrulation and pointed to Wnt3 as the main regulator acting through the canonical Wnt pathway (Rivera-Perez and Magnuson, 2005; Tortelote et al., 2013). To address this possibility, we inactivated Wnt3 in the visceral endoderm of mouse embryos while leaving two functional copies of Wnt3 in the rest of the embryo. Our results show that embryos lacking Wnt3 in the posterior visceral endoderm have delayed formation of the primitive streak but are able to continue with subsequent development. Hence, Wnt3 activity derived from the posterior visceral endoderm has a temporal role in establishing the primitive streak but its function is dispensable for embryo development. We confirmed these results using tetraploid complementation chimeras. Our data support previous chimera studies conducted by Barrow and co-workers that showed that Wnt3 in the visceral endoderm was dispensable for development of E9.5 embryos (Barrow et al., 2007). At the same time, it reveals a role for visceral endoderm-derived Wnt3 in the establishment of the primitive streak that was not evident in tetraploid complementation experiments.
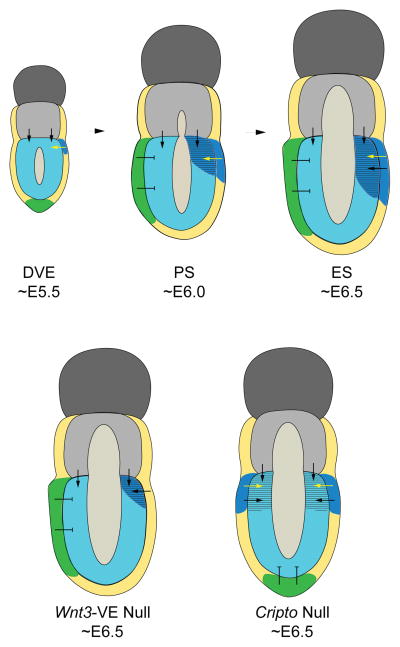
Based on the data presented here and the observations from previous studies (Rivera-Perez and Magnuson, 2005; Tortelote et al., 2013), we propose the following model for specification of the primitive streak and gastrulation in mouse embryos (Fig. 5): First, Wnt3 is initially active in the posterior visceral endoderm of embryos at the distal visceral endoderm (DVE) stage (~E5.5). This is supported by expression studies (Rivera-Perez and Magnuson, 2005) and by our current results showing that E6.5 Wnt3lacZ/lacZ mutant embryos exhibit β-gal activity in the posterior visceral endoderm but not the epiblast. Second, Wnt3 emanating from the posterior visceral endoderm induces its own expression and that of other markers for the primitive streak such as T in the epiblast. This process is underway in AVE stage (~E5.75-E6.0) embryos. Evidence for this assertion is provided by analysis of Cripto mutant embryos. Cripto mutants have radialized expression of Wnt3 only in the visceral endoderm yet they express T in the adjacent epiblast. Since T expression in the epiblast is downstream of Wnt3 (Liu, 1999, Barrow, 2007), in Cripto mutant embryos expression of T in the epiblast must depend on the activity of Wnt3 derived from the visceral endoderm. An additional source of support is provided by embryos that can initiate gastrulation despite lacking Wnt3 in the epiblast but that express Wnt3 in the posterior visceral endoderm (Tortelote et al., 2013). Third, Wnt3 activity is restricted to the posterior side of the embryo by the AVE, the source of multiple antagonists of Wnt signaling that include Sfrp1, Sfrp5 and Dkk1 (Pfister et al., 2007). This is supported by our analysis of Cripto mutant embryos. In Cripto mutants the AVE remains at the distal tip of the epiblast and T expression becomes radialized in the proximal epiblast region alongside radialization of Wnt3 in the proximal visceral endoderm. Wnt3 had previously been shown to be radialized in the proximal region of Cripto mutants, however, these studies did not determine if Wnt3 expression was restricted to the epiblast or visceral endoderm or both tissue layers of the embryo (Kimura et al., 2001). Markers of the primitive streak such as T, Fgf8 and nodal have been shown to be radialized in the proximal region of several mutants with distal AVE. This phenomenon occurs in Otx2 (Kimura et al., 2000; Perea-Gomez et al., 2001) Lpp3 (Escalante-Alcalde et al., 2003), Pten (Bloomekatz et al., 2012) and NodalD600/-- (Robertson et al., 2003) mutants. In all of these mutants, radialized expression is also accompanied by radialization of Wnt3 expression.
Fig. 5. Proposed model for primitive streak formation in the mouse.
At approximately E5.5, the DVE (green) becomes evident at the distal tip of the epiblast concomitantly with the appearance of Wnt3 expression (blue) in the posterior visceral endoderm. At ~E5.75-E6.0, Wnt3 emanating from the posterior visceral endoderm (yellow arrows) activates its own expression and that of other markers of the primitive streak, such as T (striped), in the adjacent epiblast. The antagonistic activity of the AVE prevents the spread of Wnt3 activity in the anterior epiblast. In Wnt3-VE mutants, expression of primitive streak markers is delayed appearing at late E6.5 stages. In Cripto mutants, the AVE remains at the tip of the epiblast; this allows expansion of Wnt3 expression to the anterior visceral endoderm leading to radialization of the primitive streak. The black arrows indicate potential signaling events derived from extra-embryonic ectoderm and/or posterior visceral endoderm that may work in concert with Wnt3 in the establishment of the primitive streak and maintenance of gastrulation. Abbreviations: DVE, distal visceral endoderm stage; PS, Pre-streak stage; ES, early streak stage.
The fact that Wnt3-VE mutant embryos can form a primitive streak, albeit at a delayed time, suggests that additional signaling molecules work in concert with Wnt3 to direct the formation of the primitive streak. An obvious candidate is Bmp4, a signaling molecule expressed in the extra-embryonic ectoderm abutting the proximal epiblast. Bmp4 loss of function mutations lead to gastrulation defects although some mutants can undergo gastrulation and reach somite stages (Lawson et al., 1999; Winnier et al., 1995). A role of the extra-embryonic ectoderm in primitive streak induction is also supported by the location of radialized T expression only in the proximal epiblast of Cripto mutants. Thus, signaling from the extra-embryonic ectoderm may be the source of a signal that allows Wnt3 activity derived from the visceral endoderm to activate the canonical Wnt signaling pathway in the adjacent epiblast leading to expression of primitive streak markers in the epiblast. Another possibility is that additional signaling molecules are at play in the visceral endoderm and could compensate for the absence of Wnt3. In fact, Wnt2b is expressed in the posterior visceral endoderm and epiblast of E6.5 embryos (Kemp et al., 2005). It is also interesting to note that in Otx2/Cripto double mutant embryos, T and Fgf8, are expressed in the whole epiblast even though Wnt3 expression is not detected in these embryos (Kimura et al., 2001). These studies suggest that a molecule other than Wnt3 activates these genes. Alternatively, absence of Otx2 and Cripto may lead to de-repression of these genes in the epiblast.
In conclusion, our study indicates that establishment of the primitive streak and gastrulation is a complex process that involves multiple tissue layers and multiple molecules. This view of mammalian gastrulation may help integrate the role that members of different signaling pathways such as Wnt, Tgfβ and Fgf pathways play during gastrulation.
Supplementary Material
A. Schematic representation of the targeting vector, Wnt3 locus and Wnt3lacZNeo allele. The region of homology contained in the targeting vector is indicated as thick bars and spans exons 3, 4 and a small piece of exon 5 from the NotI to the BamHI site of the Wnt3 locus. The 5′ arm of homology extends 2.5 kb and the 3′ arm 3.5 kb. IRES-lacZpA (lacZ) and PGKneobpA (neo) cassettes have been inserted into the ClaI site of exon 4. An HSV-tkpA (TK) cassette was added to the 5′ end of the construct. The neo cassette is flanked by loxP sites (yellow boxes) arranged in the same direction to allow excision with cre recombinase. B. Southern blotting of targeted ES cell clones. Genomic DNA was digested with EcoRI. The 3′ probe (black box in A) detects a 23 kb wild-type fragment and a 10.3 kb mutant fragment. Two targeted clones (numbered 14 and 15) are shown.
Highlights.
We inactivated Wnt3 in the visceral endoderm using genetics and tetraploid complementation chimeras.
Wnt3 ablation in the visceral endoderm leads to delayed development of the primitive streak.
Wnt3 function in the visceral endoderm is dispensable for embryo development.
Interplay between anterior and posterior visceral endoderm controls the position of the primitive streak.
We provide a new model to explain axial development in mammals.
Acknowledgments
We are indebted with Andy McMahon and Jeff Barrow for providing us with Wnt3c mice. We thank James Li, Michael Shen, Patrick Tam and Lorraine Robb for their generous gifts of probes. Michael Shen also kindly provided us with Cripto mutant mice. JAR-P was supported by NIH grants GM87130 and GM94874, RB by the Ben F. Love Endowment and A-KH by NIH grants HD052115 and DK084391. GT was partially supported by a fellowship from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) Brazil (GDE No. 200961/2008). Veterinary resources were supported by the NIH Cancer Center Support (Core) Grant, CA16672.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold SJ, Robertson EJ. Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nat Rev Mol Cell Biol. 2009;10:91–103. doi: 10.1038/nrm2618. [DOI] [PubMed] [Google Scholar]
- Bachvarova RF. Anterior-posterior polarization and mesoderm inducing factors in the pre-gastrula mouse embryo. Comparison to chick and mouse embryos. Adv Dev Biol. 1996;4 [Google Scholar]
- Barrow JR, Howell WD, Rule M, Hayashi S, Thomas KR, Capecchi MR, McMahon AP. Wnt3 signaling in the epiblast is required for proper orientation of the anteroposterior axis. Dev Biol. 2007;312:312–320. doi: 10.1016/j.ydbio.2007.09.030. [DOI] [PubMed] [Google Scholar]
- Barrow JR, Thomas KR, Boussadia-Zahui O, Moore R, Kemler R, Capecchi MR, McMahon AP. Ectodermal Wnt3/beta-catenin signaling is required for the establishment and maintenance of the apical ectodermal ridge. Genes Dev. 2003;17:394–409. doi: 10.1101/gad.1044903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beddington RS, Robertson EJ. Anterior patterning in mouse. Trends Genet. 1998;14:277–284. doi: 10.1016/s0168-9525(98)01499-1. [DOI] [PubMed] [Google Scholar]
- Beddington RS, Robertson EJ. Axis development and early asymmetry in mammals. Cell. 1999;96:195–209. doi: 10.1016/s0092-8674(00)80560-7. [DOI] [PubMed] [Google Scholar]
- Belaoussoff M, Farrington SM, Baron MH. Hematopoietic induction and respecification of A-P identity by visceral endoderm signaling in the mouse embryo. Development. 1998;125:5009–5018. doi: 10.1242/dev.125.24.5009. [DOI] [PubMed] [Google Scholar]
- Ben-Haim N, Lu C, Guzman-Ayala M, Pescatore L, Mesnard D, Bischofberger M, Naef F, Robertson EJ, Constam DB. The nodal precursor acting via activin receptors induces mesoderm by maintaining a source of its convertases and BMP4. Dev Cell. 2006;11:313–323. doi: 10.1016/j.devcel.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Bloomekatz J, Grego-Bessa J, Migeotte I, Anderson KV. Pten regulates collective cell migration during specification of the anterior-posterior axis of the mouse embryo. Dev Biol. 2012;364:192–201. doi: 10.1016/j.ydbio.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon F, Beddington R. Mouse gastrulation from a frog’s perspective. Semin Dev Biol. 1995;6:249–256. [Google Scholar]
- Ding J, Yang L, Yan YT, Chen A, Desai N, Wynshaw-Boris A, Shen MM. Cripto is required for correct orientation of the anterior-posterior axis in the mouse embryo. Nature. 1998;395:702–707. doi: 10.1038/27215. [DOI] [PubMed] [Google Scholar]
- Downs KM, Davies T. Staging of gastrulating mouse embryos by morphological landmarks in the dissecting microscope. Development. 1993;118:1255–1266. doi: 10.1242/dev.118.4.1255. [DOI] [PubMed] [Google Scholar]
- Escalante-Alcalde D, Hernandez L, Le Stunff H, Maeda R, Lee HS, Jr, Gang C, Sciorra VA, Daar I, Spiegel S, Morris AJ, Stewart CL. The lipid phosphatase LPP3 regulates extra-embryonic vasculogenesis and axis patterning. Development. 2003;130:4623–4637. doi: 10.1242/dev.00635. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Lewis P, Pevny L, McMahon AP. Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Mech Dev. 2002;119(Suppl 1):S97–S101. doi: 10.1016/s0925-4773(03)00099-6. [DOI] [PubMed] [Google Scholar]
- Herrmann BG. Expression pattern of the Brachyury gene in whole-mount TWis/TWis mutant embryos. Development. 1991;113:913–917. doi: 10.1242/dev.113.3.913. [DOI] [PubMed] [Google Scholar]
- Kemp C, Willems E, Abdo S, Lambiv L, Leyns L. Expression of all Wnt genes and their secreted antagonists during mouse blastocyst and postimplantation development. Dev Dyn. 2005;233:1064–1075. doi: 10.1002/dvdy.20408. [DOI] [PubMed] [Google Scholar]
- Kimura C, Shen MM, Takeda N, Aizawa S, Matsuo I. Complementary functions of Otx2 and Cripto in initial patterning of mouse epiblast. Dev Biol. 2001;235:12–32. doi: 10.1006/dbio.2001.0289. [DOI] [PubMed] [Google Scholar]
- Kimura C, Yoshinaga K, Tian E, Suzuki M, Aizawa S, Matsuo I. Visceral endoderm mediates forebrain development by suppressing posteriorizing signals. Dev Biol. 2000;225:304–321. doi: 10.1006/dbio.2000.9835. [DOI] [PubMed] [Google Scholar]
- Kwon GS, Hadjantonakis AK. Transthyretin mouse transgenes direct RFP expression or Cre-mediated recombination throughout the visceral endoderm. Genesis. 2009;47:447–455. doi: 10.1002/dvg.20522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon GS, Viotti M, Hadjantonakis AK. The endoderm of the mouse embryo arises by dynamic widespread intercalation of embryonic and extraembryonic lineages. Dev Cell. 2008;15:509–520. doi: 10.1016/j.devcel.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson KA, Dunn NR, Roelen BA, Zeinstra LM, Davis AM, Wright CV, Korving JP, Hogan BL. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 1999;13:424–436. doi: 10.1101/gad.13.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- Mishina Y, Suzuki A, Ueno N, Behringer RR. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev. 1995;9:3027–3037. doi: 10.1101/gad.9.24.3027. [DOI] [PubMed] [Google Scholar]
- Nagy A. Manipulating the mouse embryo: a laboratory manual. 3. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, N.Y: 2003. [Google Scholar]
- Perea-Gomez A, Lawson KA, Rhinn M, Zakin L, Brulet P, Mazan S, Ang SL. Otx2 is required for visceral endoderm movement and for the restriction of posterior signals in the epiblast of the mouse embryo. Development. 2001;128:753–765. doi: 10.1242/dev.128.5.753. [DOI] [PubMed] [Google Scholar]
- Pfister S, Steiner KA, Tam PP. Gene expression pattern and progression of embryogenesis in the immediate post-implantation period of mouse development. Gene Expr Patterns. 2007;7:558–573. doi: 10.1016/j.modgep.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Rivera-Perez JA, Jones V, Tam PP. Culture of whole mouse embryos at early postimplantation to organogenesis stages: developmental staging and methods. Methods Enzymol. 2010;476:185–203. doi: 10.1016/S0076-6879(10)76011-0. [DOI] [PubMed] [Google Scholar]
- Rivera-Perez JA, Magnuson T. Primitive streak formation in mice is preceded by localized activation of Brachyury and Wnt3. Dev Biol. 2005;288:363–371. doi: 10.1016/j.ydbio.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Robertson EJ, Norris DP, Brennan J, Bikoff EK. Control of early anterior-posterior patterning in the mouse embryo by TGF-beta signalling. Philos Trans R Soc Lond B Biol Sci. 2003;358:1351–1357. doi: 10.1098/rstb.2003.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez TA, Casey ES, Harland RM, Smith JC, Beddington RS. Distinct enhancer elements control Hex expression during gastrulation and early organogenesis. Dev Biol. 2001;234:304–316. doi: 10.1006/dbio.2001.0265. [DOI] [PubMed] [Google Scholar]
- Stern CD. Gastrulation: From cells to embryo. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, N. Y: 2004. [Google Scholar]
- Stern CD, Charite J, Deschamps J, Duboule D, Durston AJ, Kmita M, Nicolas JF, Palmeirim I, Smith JC, Wolpert L. Head-tail patterning of the vertebrate embryo: one, two or many unresolved problems? Int J Dev Biol. 2006;50:3–15. doi: 10.1387/ijdb.052095cs. [DOI] [PubMed] [Google Scholar]
- Sundararajan S, Wakamiya M, Behringer RR, Rivera-Perez JA. A fast and sensitive alternative for beta-galactosidase detection in mouse embryos. Development. 2012;139:4484–4490. doi: 10.1242/dev.078790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortelote GG, Hernandez-Hernandez JM, Quaresma AJ, Nickerson JA, Imbalzano AN, Rivera-Perez JA. Wnt3 function in the epiblast is required for the maintenance but not the initiation of gastrulation in mice. Dev Biol. 2013;374:164–173. doi: 10.1016/j.ydbio.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay KD, Hoodless PA, Bikoff EK, Robertson EJ. Formation of the definitive endoderm in mouse is a Smad2-dependent process. Development. 2000;127:3079–3090. doi: 10.1242/dev.127.14.3079. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, Bhatt S, Herrmann BG. Expression pattern of the mouse T gene and its role in mesoderm formation. Nature. 1990;343:657–659. doi: 10.1038/343657a0. [DOI] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. Schematic representation of the targeting vector, Wnt3 locus and Wnt3lacZNeo allele. The region of homology contained in the targeting vector is indicated as thick bars and spans exons 3, 4 and a small piece of exon 5 from the NotI to the BamHI site of the Wnt3 locus. The 5′ arm of homology extends 2.5 kb and the 3′ arm 3.5 kb. IRES-lacZpA (lacZ) and PGKneobpA (neo) cassettes have been inserted into the ClaI site of exon 4. An HSV-tkpA (TK) cassette was added to the 5′ end of the construct. The neo cassette is flanked by loxP sites (yellow boxes) arranged in the same direction to allow excision with cre recombinase. B. Southern blotting of targeted ES cell clones. Genomic DNA was digested with EcoRI. The 3′ probe (black box in A) detects a 23 kb wild-type fragment and a 10.3 kb mutant fragment. Two targeted clones (numbered 14 and 15) are shown.