Abstract
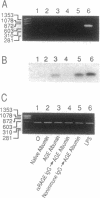
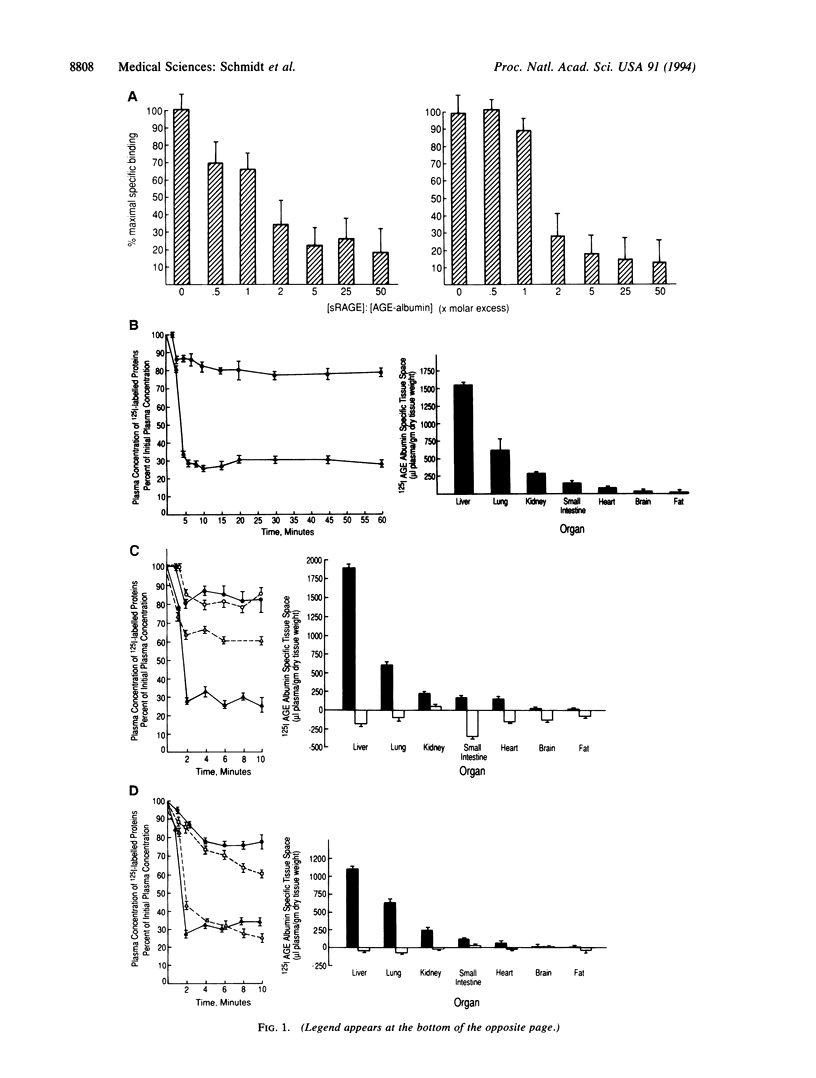
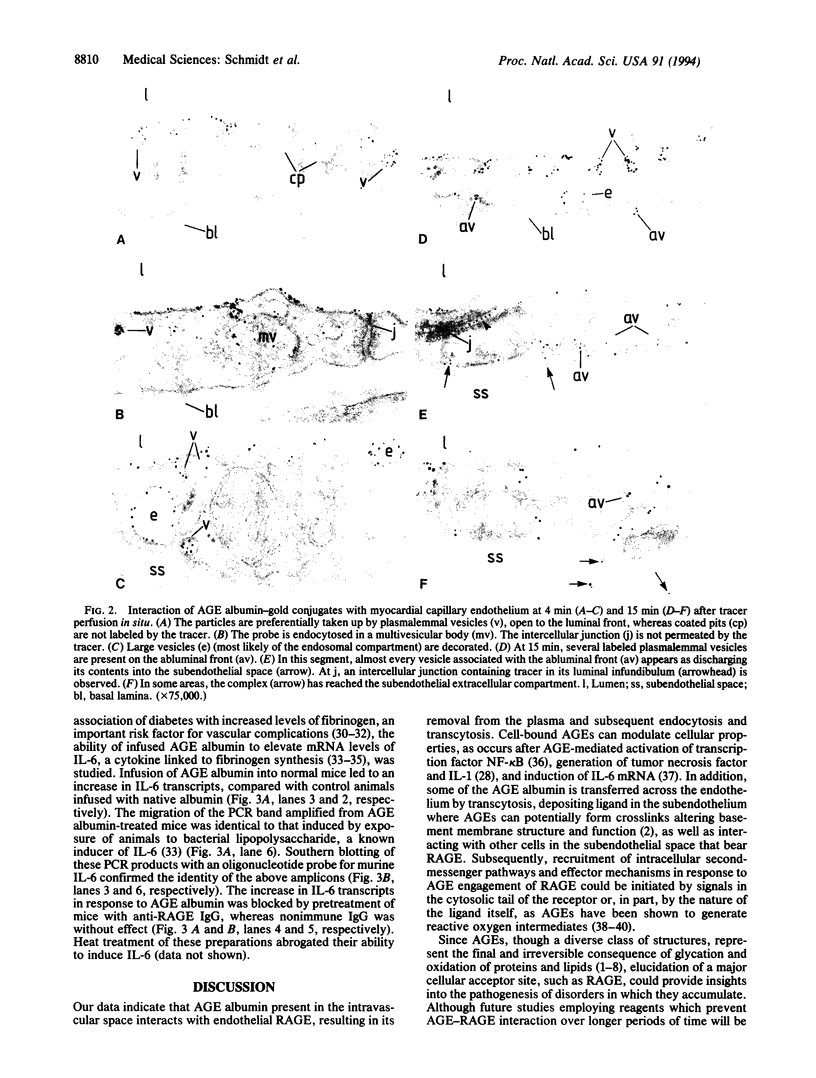
The extended interaction of aldoses with proteins or lipids results in nonenzymatic glycation and oxidation, ultimately forming AGEs, the presence of which in the plasma and vessel wall is associated with diabetic vascular complications. We show here that AGE albumin in the intravascular space interacts with the vessel wall via binding to an integral membrane protein, receptor for AGE (RAGE), a member of the immunoglobulin superfamily, resulting in clearance from the plasma and induction of interleukin 6 mRNA. Intravenously infused 125I-AGE albumin showed a rapid phase of plasma clearance with deposition in several organs. Rapid removal of 125I-AGE albumin from the plasma was prevented by administration of a soluble, truncated form of RAGE, which blocked binding of 125I-labeled AGE albumin to cultured endothelial cells and mononuclear phagocytes, as well as by pretreatment with anti-RAGE IgG. Ultrastructural studies with AGE albumin-colloidal gold conjugates perfused in situ showed that in murine coronary vasculature this probe was taken up by endothelial plasmalemmal vesicles followed by transport either to the abluminal surface or by accumulation in intracellular vesicular structures reminiscent of endosomes and lysosomes. Consequences of AGE-RAGE interaction included induction of interleukin 6 mRNA expression in mice. These data indicate that RAGE mediates the interaction of AGEs with the vessel wall, both for removal of these glycated proteins from the plasma and for changes in gene expression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baynes J. W. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991 Apr;40(4):405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- Brett J., Schmidt A. M., Yan S. D., Zou Y. S., Weidman E., Pinsky D., Nowygrod R., Neeper M., Przysiecki C., Shaw A. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am J Pathol. 1993 Dec;143(6):1699–1712. [PMC free article] [PubMed] [Google Scholar]
- Brownlee M., Cerami A., Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988 May 19;318(20):1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- Bucala R., Makita Z., Koschinsky T., Cerami A., Vlassara H. Lipid advanced glycosylation: pathway for lipid oxidation in vivo. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6434–6438. doi: 10.1073/pnas.90.14.6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Borden P., Liao J., Kabat E. A. Variable region cDNA sequences of three mouse monoclonal anti-idiotypic antibodies specific for anti-alpha(1----6)dextrans with groove- or cavity-type combining sites. Mol Immunol. 1992 Sep;29(9):1121–1129. doi: 10.1016/0161-5890(92)90045-y. [DOI] [PubMed] [Google Scholar]
- Choi S. Y., Fong L. G., Kirven M. J., Cooper A. D. Use of an anti-low density lipoprotein receptor antibody to quantify the role of the LDL receptor in the removal of chylomicron remnants in the mouse in vivo. J Clin Invest. 1991 Oct;88(4):1173–1181. doi: 10.1172/JCI115419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller B. S., Frank R. N., Milton R. C., Gralnick H. R. Plasma cofactors of platelet function: correlation with diabetic retinopathy and hemoglobins Ala-c. Ann Intern Med. 1978 Mar;88(3):311–316. doi: 10.7326/0003-4819-88-3-311. [DOI] [PubMed] [Google Scholar]
- David G. S., Reisfeld R. A. Protein iodination with solid state lactoperoxidase. Biochemistry. 1974 Feb 26;13(5):1014–1021. doi: 10.1021/bi00702a028. [DOI] [PubMed] [Google Scholar]
- Dyer D. G., Blackledge J. A., Thorpe S. R., Baynes J. W. Formation of pentosidine during nonenzymatic browning of proteins by glucose. Identification of glucose and other carbohydrates as possible precursors of pentosidine in vivo. J Biol Chem. 1991 Jun 25;266(18):11654–11660. [PubMed] [Google Scholar]
- Dyer D. G., Dunn J. A., Thorpe S. R., Bailie K. E., Lyons T. J., McCance D. R., Baynes J. W. Accumulation of Maillard reaction products in skin collagen in diabetes and aging. J Clin Invest. 1993 Jun;91(6):2463–2469. doi: 10.1172/JCI116481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito C., Gerlach H., Brett J., Stern D., Vlassara H. Endothelial receptor-mediated binding of glucose-modified albumin is associated with increased monolayer permeability and modulation of cell surface coagulant properties. J Exp Med. 1989 Oct 1;170(4):1387–1407. doi: 10.1084/jem.170.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller J. H., Keen H., Jarrett R. J., Omer T., Meade T. W., Chakrabarti R., North W. R., Stirling Y. Haemostatic variables associated with diabetes and its complications. Br Med J. 1979 Oct 20;2(6196):964–966. doi: 10.1136/bmj.2.6196.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganda O. P., Arkin C. F. Hyperfibrinogenemia. An important risk factor for vascular complications in diabetes. Diabetes Care. 1992 Oct;15(10):1245–1250. doi: 10.2337/diacare.15.10.1245. [DOI] [PubMed] [Google Scholar]
- Ghinea N., Fixman A., Alexandru D., Popov D., Hasu M., Ghitescu L., Eskenasy M., Simionescu M., Simionescu N. Identification of albumin-binding proteins in capillary endothelial cells. J Cell Biol. 1988 Jul;107(1):231–239. doi: 10.1083/jcb.107.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitescu L., Fixman A., Simionescu M., Simionescu N. Specific binding sites for albumin restricted to plasmalemmal vesicles of continuous capillary endothelium: receptor-mediated transcytosis. J Cell Biol. 1986 Apr;102(4):1304–1311. doi: 10.1083/jcb.102.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich P. C., Castell J. V., Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990 Feb 1;265(3):621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisberger M., Rosset J. Colloidal gold, a useful marker for transmission and scanning electron microscopy. J Histochem Cytochem. 1977 Apr;25(4):295–305. doi: 10.1177/25.4.323352. [DOI] [PubMed] [Google Scholar]
- Kirstein M., Brett J., Radoff S., Ogawa S., Stern D., Vlassara H. Advanced protein glycosylation induces transendothelial human monocyte chemotaxis and secretion of platelet-derived growth factor: role in vascular disease of diabetes and aging. Proc Natl Acad Sci U S A. 1990 Nov;87(22):9010–9014. doi: 10.1073/pnas.87.22.9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T., Hirano T. Molecular regulation of B lymphocyte response. Annu Rev Immunol. 1988;6:485–512. doi: 10.1146/annurev.iy.06.040188.002413. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McCance D. R., Dyer D. G., Dunn J. A., Bailie K. E., Thorpe S. R., Baynes J. W., Lyons T. J. Maillard reaction products and their relation to complications in insulin-dependent diabetes mellitus. J Clin Invest. 1993 Jun;91(6):2470–2478. doi: 10.1172/JCI116482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullarkey C. J., Edelstein D., Brownlee M. Free radical generation by early glycation products: a mechanism for accelerated atherogenesis in diabetes. Biochem Biophys Res Commun. 1990 Dec 31;173(3):932–939. doi: 10.1016/s0006-291x(05)80875-7. [DOI] [PubMed] [Google Scholar]
- Neeper M., Schmidt A. M., Brett J., Yan S. D., Wang F., Pan Y. C., Elliston K., Stern D., Shaw A. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992 Jul 25;267(21):14998–15004. [PubMed] [Google Scholar]
- Predescu D., Simionescu M., Simionescu N., Palade G. E. Binding and transcytosis of glycoalbumin by the microvascular endothelium of the murine myocardium: evidence that glycoalbumin behaves as a bifunctional ligand. J Cell Biol. 1988 Nov;107(5):1729–1738. doi: 10.1083/jcb.107.5.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman N. B., Williamson J. R., Brownlee M. Glucose and diabetic vascular disease. FASEB J. 1992 Aug;6(11):2905–2914. doi: 10.1096/fasebj.6.11.1644256. [DOI] [PubMed] [Google Scholar]
- Sakurai T., Sugioka K., Nakano M. O2- generation and lipid peroxidation during the oxidation of a glycated polypeptide, glycated polylysine, in the presence of iron-ADP. Biochim Biophys Acta. 1990 Mar 12;1043(1):27–33. doi: 10.1016/0005-2760(90)90106-8. [DOI] [PubMed] [Google Scholar]
- Schmidt A. M., Vianna M., Gerlach M., Brett J., Ryan J., Kao J., Esposito C., Hegarty H., Hurley W., Clauss M. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J Biol Chem. 1992 Jul 25;267(21):14987–14997. [PubMed] [Google Scholar]
- Schmidt A. M., Yan S. D., Brett J., Mora R., Nowygrod R., Stern D. Regulation of human mononuclear phagocyte migration by cell surface-binding proteins for advanced glycation end products. J Clin Invest. 1993 May;91(5):2155–2168. doi: 10.1172/JCI116442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer J. E., Carley W. W., Palade G. E. Albumin interacts specifically with a 60-kDa microvascular endothelial glycoprotein. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6773–6777. doi: 10.1073/pnas.85.18.6773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell D. R., Monnier V. M. Structure elucidation of a senescence cross-link from human extracellular matrix. Implication of pentoses in the aging process. J Biol Chem. 1989 Dec 25;264(36):21597–21602. [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J. Sizing of protein A-colloidal gold probes for immunoelectron microscopy. J Cell Biol. 1981 Aug;90(2):533–536. doi: 10.1083/jcb.90.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spady D. K., Bilheimer D. W., Dietschy J. M. Rates of receptor-dependent and -independent low density lipoprotein uptake in the hamster. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3499–3503. doi: 10.1073/pnas.80.11.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassara H., Brownlee M., Manogue K. R., Dinarello C. A., Pasagian A. Cachectin/TNF and IL-1 induced by glucose-modified proteins: role in normal tissue remodeling. Science. 1988 Jun 10;240(4858):1546–1548. doi: 10.1126/science.3259727. [DOI] [PubMed] [Google Scholar]
- Yan S. D., Schmidt A. M., Anderson G. M., Zhang J., Brett J., Zou Y. S., Pinsky D., Stern D. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem. 1994 Apr 1;269(13):9889–9897. [PubMed] [Google Scholar]
- Yang Z., Makita Z., Horii Y., Brunelle S., Cerami A., Sehajpal P., Suthanthiran M., Vlassara H. Two novel rat liver membrane proteins that bind advanced glycosylation endproducts: relationship to macrophage receptor for glucose-modified proteins. J Exp Med. 1991 Sep 1;174(3):515–524. doi: 10.1084/jem.174.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. H., Lin J. X., Vilcek J. Interleukin-6 induction by tumor necrosis factor and interleukin-1 in human fibroblasts involves activation of a nuclear factor binding to a kappa B-like sequence. Mol Cell Biol. 1990 Jul;10(7):3818–3823. doi: 10.1128/mcb.10.7.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]