Introduction
Cochlear implants (CIs) restore sound perception to individuals with severe to profound hearing loss (HL) by using electrical current to directly stimulate surviving auditory neurons (spiral ganglion neurons, SGNs). Shallow insertion of a shorter CI array at the base of the cochlea allows preservation of functional cochlear structures towards the apex. These hearing preservation CIs, which vary in length, are designed to both preserve residual acoustic hearing and provide electrical speech processing in the same ear1,2. In clinical trials, the major advantage of electric plus acoustic stimulation (EAS) lies in its ability to significantly enhance users’ understanding of speech in the presence of background noise and recognition of musical melodies as compared to users of the standard length electrode arrays3,4,5,6,7. Localization of sound is also improved when acoustic hearing is preserved in both ears8. These benefits hold for patients with all but the most profound levels of HL9. These findings have been attributed to the finer frequency resolution of the residual acoustic hearing10.
With meticulous surgical technique and appropriate electrode arrays, the ability to preserve functional acoustic hearing remains high11. Relatively mild ipsilateral post-implantation HL (~12 dB PTA) occurs with insertion2,12,13. In addition, there appears to be a subset of patients that experience delayed HL that occurs after device activation, or one to six months after implantation14.
In order to develop a better understanding of post-implantation HL, we first evaluated five index subjects that were implanted at our center and developed significant late hearing loss. We found that in these five subjects, high maximum electrical charge coincided with delayed HL. To further clarify the effect of high electrical charge on late HL in this cohort, we conducted additional retrospective analysis of all hearing preservation implantees at our center. We then examined a more detailed time course of ipsilateral HL after hearing preservation cochlear implantation in all patients from the Nucleus S8 multicenter FDA trial, the largest collection of hearing preservation patients to date.
Based on these observations, we hypothesize that excitotoxicity from EAS may cause delayed post-implantation HL in a manner similar to noise induced HL. To begin to determine which cochlear structures are most suspectible to excessive electrical stimulation (ES), we analyzed the consequences of high voltage stimulation to rat organotypic cochlear explants.
Materials and Methods
Subjects
Case review
Audiometric and programming data were reviewed for five patients who were noted to experience significant acoustic threshold shifts 1–6 months after implantation. It was recognized that all five had their ES maximal comfort or C levels set at a high intensity with short duration pulse-width. These subjects, implanted at our center between 2005 and 2009, met criteria for hearing preservation array implantation as previously described2. Demographic and device characteristics are detailed in Table 1. All had minimal change in pure tone average (PTA) frequencies after implantation prior to implant activation. Clinically derived C levels were converted to overall electrical charge (nanoCoulombs) to correct for variations in pulse-width. The following equations were used:
| (1) |
| (2) |
Normative data regarding electrode charge was abstracted from both Nucleus Contour Advance perimodiolar standard-length electrode arrays, which were implanted through a scala tympani cochleostomy (N = 47), and Nucleus 422 Slim Straight electrode arrays, implanted through a round window approach (N=16). Both populations met standard implantation criteria with severe to profound HL (>70dB loss) at 250 Hz and above.
Table 1.
Demographics of index patients.
| Subject | Device | Device characteristics |
Gender | Age | Etiology of HL |
|---|---|---|---|---|---|
| T1 | S12 | 10mm, 10 channels | M | 60 | Noise |
| T2 | S12 | 10mm, 10 channels | F | 60 | Unknown |
| T3 | S12 | 10mm, 10 channels | M | 65 | Hereditary |
| A1 | S8 | 10mm, 6 channel | F | 59 | Noise |
| L1 | L24 | 16mm, 22 channels | M | 23 | Hereditary |
Retrospective cohort study
All hearing preservation cochlear implant recipients at the University of Iowa from 1999 to 2013 were considered for review. Patients with a relapsing etiology of hearing loss (e.g. autoimmune) were excluded from analysis (n=2). Patients with insufficient serial audiograms and mapping parameters for comparison were also excluded (n=2). 42 of 46 hearing preservation implantees at our center met criteria for inclusion. S8 (n=19), S12 (n=11), L24 (n=12). Average pre-activation thresholds did not vary between these groups. The total population comprised 20 males and 22 females, aged between 23 to 80 years at the time of implantation (mean=56). Duration of high frequency HL ranged from 5 to 57 years (mean = 24). Etiology of HL was categorized as follows: hereditary (35%), noise induced (30%), infectious (3%), and unknown (32%). Hearing loss, maximum current levels and pulse widths from activation to 12 months post-activation were examined for univariate analysis and generation of an odds ratio. Maximum charge was calculated as described above for each functional electrode and averaged across the electrode array. The highest level of electrical charge prior to or concomitant with the peak post-activation rate of HL was included for statistical analysis. In all instances, those with elevated peak charge had consistently elevated charge subsequently throughout the study period.
Natural history
In addition, audiometric data of 85 patients who underwent Nucleus Hybrid S8 cochlear implantation as part of the adult Food and Drug Administration multi-center clinical trial in the USA from 1999 to 2009 were reviewed. All participants had severe to profound HL above 2000 Hz and were implanted in the worse hearing ear. Detailed candidacy information is described in Gantz et al. 2009. The total population comprised 39 males and 46 females, aged between 19 and 82 years at the time of implantation (mean = 59). Duration of high frequency HL ranged from 3 to 66 years (mean = 26). Etiology of HL was categorized as follows: hereditary (31%), noise induced (20%), infectious (5%), unknown (42%) and other (2%). Patients with HL of a relapsing type i.e. autoimmune were omitted from the study (n=2). Patients with device failure, electrode extrusion, and/or confounding symptoms of inner ear disorders (e.g. episodic vertigo) were also excluded.
Audiometry
Audiometric data consisted of standard PTAs collected pre-operatively, at activation (1 month postoperatively) and at 4, 6 and 12 months after implantation. Frequencies tested included 125, 250, 500, 750 and 1000 Hz bilaterally. For each ear, threshold was averaged across frequency at each audiogram time-point to simplify further analyses. Threshold shift at each time point was assayed by subtracting pre-operative average threshold from the last post-operative average threshold at that time point. Contralateral threshold shift was then subtracted in order to correct for natural progression of pre-morbid disease in the implanted ear. These calculations were used to generate an ear-specific frequency-averaged measure of threshold shift. Appreciable hearing threshold was defined as an absolute (uncorrected) threshold of 100dB or less.
Organotypic Cultures
The institutional animal care and use committee at the University of Iowa approved all protocols used in this study. Organotypic cochlear explant cultures including the organ of Corti (OOC) and spiral ganglion were prepared from post natal day 5 (P5) rat pups15,16. P5 rat cochleae of both sexes were dissected in ice-cold PBS. The membranous labyrinth was separated from the modiolus and the stria vascularis and spiral ligament were carefully removed. With the organ of Corti and spiral ganglion kept intact, Reissner’s membrane and tectorial membrane were removed with fine forceps in order to provide an in vitro model of the cochlear sensory epithelium with intact synaptic contacts. Explants were cultured in 5% FBS DMEM/N2 media for 2–3 hours in a modified Warner chamber (Warner Instruments, Hamden CT). Explants were then subjected to either 3 hours of high-voltage patterned ES, depolarizing media (80 mmol K+, 80K) as a positive control, or nondepolarizing media (5.4 mmol K+) as a negative control. Electrical stimuli comprised 40 ms biphasic pulses presented at 10 V amplitude with a frequency of 10Hz for three hours. Impedance of the culture system was 1.1 kOhm as measured with a Grass electrode impedance meter, Model EZM5 (Astro-Med, Inc., W. Warwick, RI). Thus, the overall charge delivered with this system was 0.36 µC/phase, roughly an order of magnitude greater (10×) than that delivered by CIs. Preliminary experiments indicated that 3V and 5V amplitude stimulation at 10 Hz for three hours did not cause a discernable loss of hair cells or neuron/neurite damage. Fixation occurred at 24 hours. Cultures were then immunolabelled with rabbit anti-Myosin VIIa antibody as a marker of hair cells and mouse neurofilament 200 (NF200) antibody as a marker of spiral ganglion neurons (SGNs). Nuclei were stained with DAPI. Images of cochlear explants were captured using a Leica SP5 confocal microscope (Buffalo Grove, IL).
Hair cell and spiral ganglion neurite density counts
Inner and outer hair cell (IHC and OHC) density counts were performed from reconstructed z-stacks for the middle turn of the cochlea and expressed as number of HC/mm along the long axis of the organ of Corti. In some circumstances, the morphology of the tissue was distorted and it was not possible to distinguish inner and outer HCs (e.g. in 80K). In these cases total HC counts were obtained. The number of NF200-positive SGN peripheral neurites crossing a line drawn at 100 mm from the OOC were counted and expressed as the number of neurites/mm. HC and neurite density counts were performed on at least 12 explants derived from at least 3 separate experiments.
Statistical Analysis
All error bars were calculated using standard error of the mean (SEM). Differences in hearing threshold variations between groups were evaluated with Student’s t-tests after assessing for normality using K-S tests. Differences in explants (i.e. HC and neurite density) were analyzed in an identical fashion. Pearson product moment correlation was used for regression analysis of rate of hearing loss as a function of maximum charge exposure. Odds ratio was calculated with a p value derived by Fisher’s exact test.
Results
Five index patients alerted us to the possibility of loss of residual hearing secondary to high levels of ES. All had high maximum electrical charge levels either at activation or prior to their most significant loss. HL at one year ranged from 31 to 52 dB when averaged across frequency (125 to 1000 Hz) and corrected for contralateral loss as described above. All five suffered their most significant HL after activation. Figure 1 depicts the maximum charge settings for each electrode worn at the time of the audiogram that identified the greatest change in threshold for each patient, as compared to normative charge levels from patients with Nucleus Contour Advance perimodiolar standard-length electrode arrays and Nucleus 422 slim straight standard-length arrays. These settings, derived from clinical comfort level testing for daily use based on psychophysical loudness scaling for adequate audibility, were significantly higher and indeed almost double normative settings based on long electrode CI users.
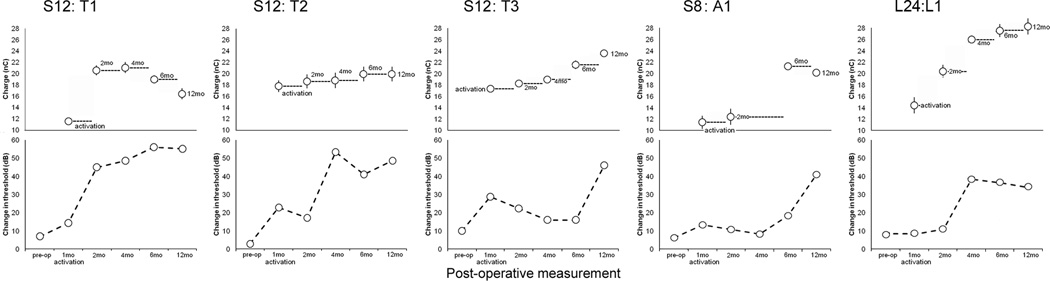
Figure 1.
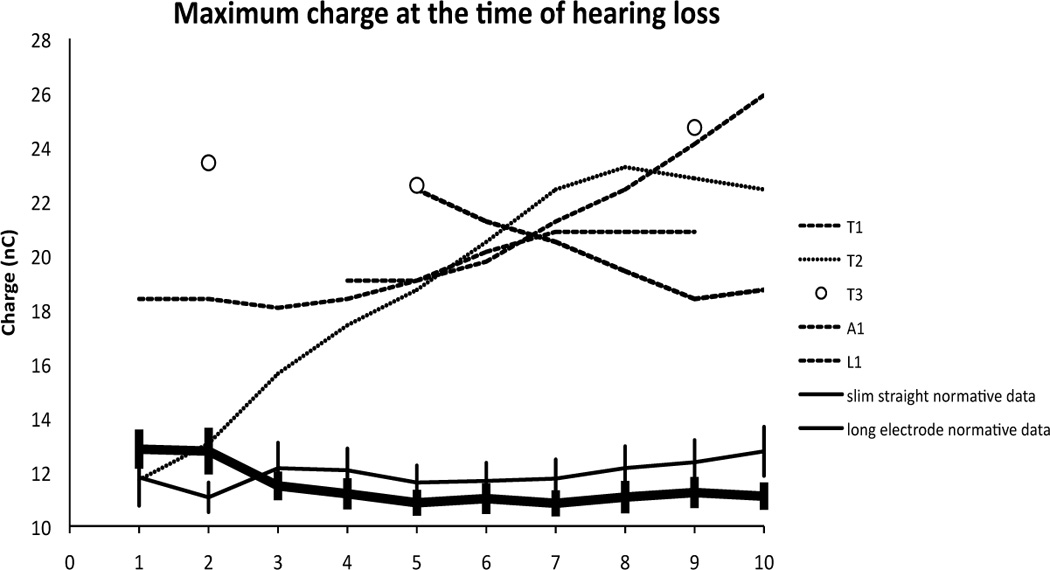
High levels of electrical charge were noted across electrodes in five patients at the time of maximal HL. Maximum charge level (nanoCoulomb) is derived from the pulse width and clinical level for maximum comfort that each patient was wearing at the time of their greatest drop in hearing. Subject A1 was a participant in the S8 FDA clinical trial and wore a 6 electrode array that has been spatially corrected to compare with T1, T2 and T3. The latter are participants in the S12 FDA trial and have 10 electrode Hybrid arrays. L1 is a participant in the L24 FDA clinical trial and wears a 22 electrode array. Normative data was averaged from 47 standard-length Nucleus Contour Advance electrode CI users implanted via a scala tympani cochleostomy and 16 standard-length Nucleus 422 slim straight CI users implanted via the round window – both groups with no residual hearing. Error bars represent standard error of the mean (SEM).
A direct comparison between serial audiograms and maximum charge levels for each of these five patients (Figure 2) revealed that each experienced significant HL either subsequent to or coincident with implementation of high clinical settings for maximum charge. Of note, all but T1 and L1 had some recovery from the initial post-implantation HL prior to the onset of ES. T3 and A1 had stable thresholds until the current levels were elevated at the 6-month programming session. L1 experienced a large threshold shift after high current levels were permitted at the 2-month programming session. T1 also had the highest current levels at the beginning, which may not have allowed for recovery of post-implantation HL. In summary, while a common pattern of HL and current level is not apparent among these patients, each patient was exposed to high current levels and suffered significant HL after activation of the CI. In the case of L1 most of the HL took place before the patient was exposed to high charge stimulation but in the other four cases significant HL happened after high charge stimulation was introduced.
Figure 2.
Hearing loss occurred subsequent to or coincident with high charge in these index patients. Serial audiograms are depicted underneath serial mapping parameters for each patient. Each data point above is derived from maximum comfort level worn for daily use at the time of the corresponding audiogram averaged across all electrodes (Error bars = SEM). These levels are then converted to nanoCoulombs to compare charge levels directly accounting for both stimulus amplitude and pulse width.
Retrospective cohort analysis
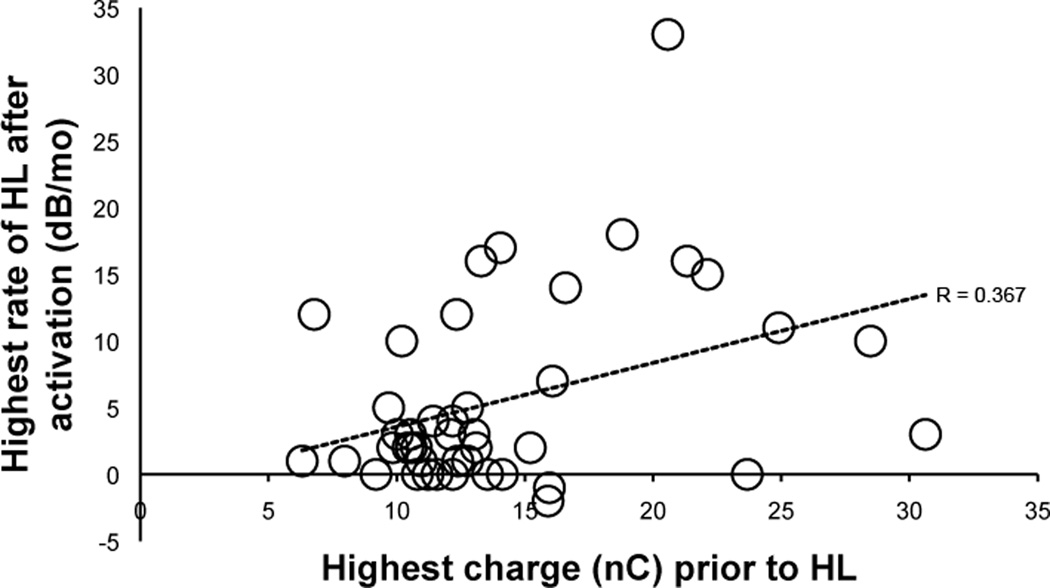
The data from the above group of subjects led us to analyze the rate of HL after activation as a function of the highest level of maximum permitted charge in all patients implanted with a hearing preservation array at our center. When comparing the highest rate of hearing loss after activation with peak maximum charge levels, the Pearson correlation coefficient was 0.366 (p=0.016) indicating a significant, moderate association of hearing loss with stimulation charge (Figure 3). Next, we divided the patients into groups based on the rate of hearing loss after activation(≥10dB/mo or <10dB/mo) and maximum charge (≥16nC or <16nC). This charge level is 1 nC above the higher limit of the 95% confidence interval for charge in the patients with standard long electrode (Nucleus Contour Advance) or slim straight (Nucleus 422) arrays (range 11.5–15) used as normative controls in Figure 1. Of the 42 hearing preservation cochlear implantees that met inclusion criteria, seven experienced a rapid rate of ipsilateral hearing loss (≥10dB/mo) subsequent to or concurrent with high maximum charge (≥16nC); three individuals experienced no acceleration after high charge exposure, four individuals had a high rate of hearing loss with no significant charge exposure while 28 individuals had stable hearing in the absence of high charge (Table 2). Thus, the odds ratio for a rapid rate of hearing loss after exposure to high charge is 16.3 – further confirming a robust and statistically significant association (Fisher’s exact test, p<0.01).
Figure 3.
Linear regression of peak rate of HL (dB/mo) as a function of peak electrical charge level (nC) during he first 12 months of stimulation reveals a modest but significant association (Pearson = 0.366, p=0.016).
Table 2.
Odds ratio for hearing loss after high charge
| HL ≥10dB/mo | HL<10dB/mo | n | |
|---|---|---|---|
| Charge ≥16nC | 7 | 3 | 10 |
| Charge <16nC | 4 | 28 | 32 |
| n | 11 | 31 | 42 |
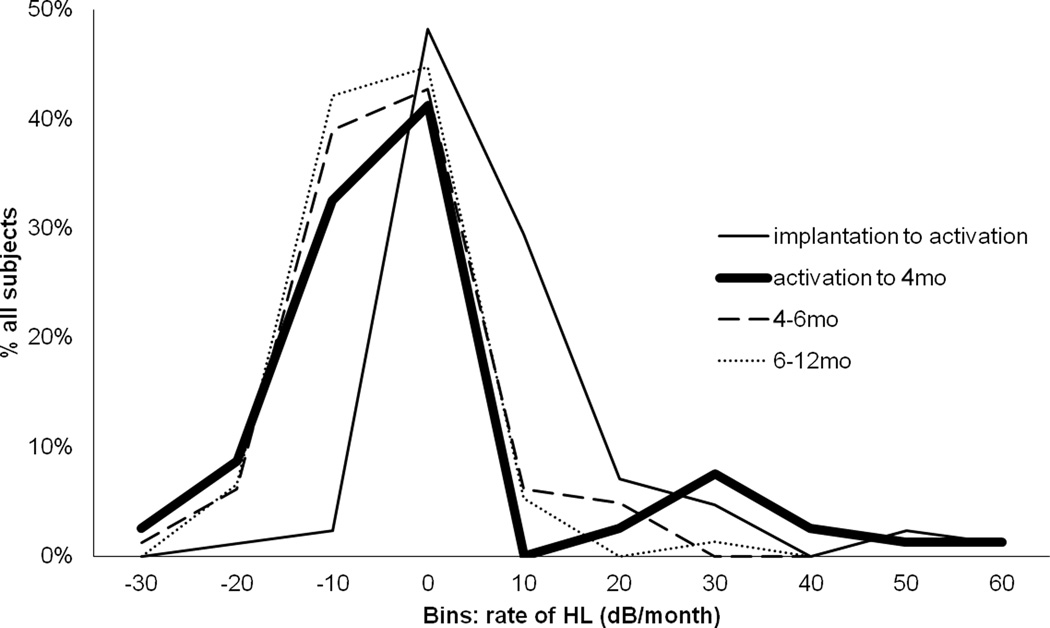
We then carefully examined the temporal pattern of HL in the patients from the Hybrid S8 trial. At least 3 sequential audiograms were performed on 82 of 85 subjects to allow for comparison of HL progression over time. Consistent with prior reports, high rates of hearing preservation are associated with Hybrid S8 implantation. Hybrid S8 recipients demonstrated an average of 12.2 dB threshold shift one month following implantation, 1.2 dB/month in the 3 months after activation, 0.2 dB/month from 3 to 6 months post activation, and 0.1 dB/month from 6 to 12 months post-activation (bold line in Figure 4). Thus, preserved hearing typically remains stable after an initial mild post-operative HL.
Figure 4.
A small subset of patients had high rates of HL after activation. A frequency histogram of rate of HL shows that the majority of patients had rapid loss between implantation and activation (~1 month post-operatively). For most of these patients rate of HL then decreased or plateau-ed. The bold line depicts rate of HL from activation to 4 months post-operatively - at the onset of ES. Here we see a bimodal distribution in that a small proportion of patients had an increase in HL rate at this time.
Consistent with this finding, a frequency histogram of rate of HL of the implanted ear with respect to the contralateral for individual subjects reveals that during the period from implantation to activation, patients had the broadest range of HL with net threshold increase occurring in the majority of patients (Figure 4, thin solid line). 65 of 82 (79.3%) had a mild 12 dB threshold change associated with implantation and identified at one month post operative just prior to activation of the implant. In contrast, from activation to 4 months post-operatively, most patient remained stable or improved with respect to the contralateral ear (Figure 4, bold line). 15% however, experience HL acceleration >10dB/month during this time interval that is denoted by a separate peak. No such separable peaks are noted at later time intervals (Figure 4, dashed lines).
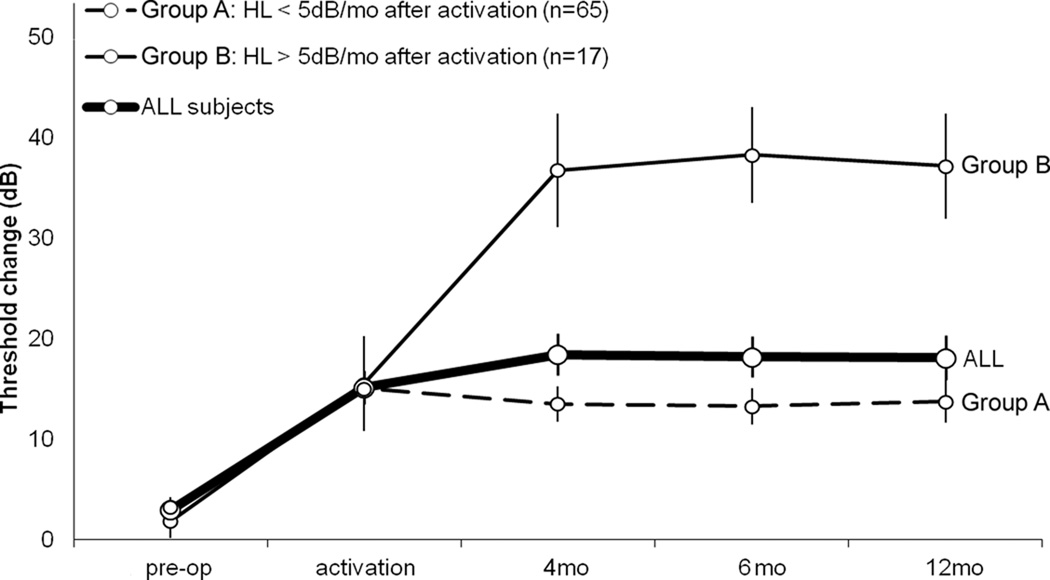
Two groups of subjects could be identified following activation of the implant and hearing aid in the same ear. Group A subjects experienced threshold changes of less than 5 dB/month after activation and ranged from a decrease of 24 dB to an increase of 27 dB (mean = −1 +/− 1.2 dB). After activation, hearing in these ears subsequently improved (mean threshold shift: −7 dB, n=39), remained the same (0 dB, n=7) or continued to worsen only slightly (11 dB, n=19). Group B subjects, in contrast, 17 of 82 (20.5%), experienced more rapid HL (>5 dB/month) after activation of the CI. Group B subjects experienced absolute threshold changes ranging from a decrease of 19 dB to an increase of 64 dB (mean = 24 +/− 4.6 dB) in the implanted ear. Ten of these subjects experienced greater than 30 dB PTA threshold shift. Figure 5 depicts the time course of threshold shifts for groups stratified by rate of HL above (group B, thin solid line; n=17) or below (group A, dashed line; n=65) 5 dB/month after activation for all subjects (ALL, thick solid line; n=82). No difference was seen in threshold shift between these two groups from the pre-operative audiogram to activation suggesting the possibility that activation of the implant may be a critical turning point for these patients.
Figure 5.
Acute post-implantation HL (pre-op to activation) was uniform irrespective of post-activation loss. The time course of threshold shifts for groups were stratified by rate of HL after activation, i.e. when stratified into groups that had more or less than 5 dB/month of HL after activation (n=65 and 17 respectively). No difference was seen in threshold shift between these two groups from the pre-op audiogram to activation suggesting the possibility that activation of the implant may be a critical turning point for these patients. As depicted by the bold line, on average implantees lost 12.2 dB in the month after implantation, 1.2 dB/month in the 3 months after activation (4 months post-implantation), 0.2 dB/month from 4 to 6 months post operatively, and 0.1 dB/month from 6 to 12 months post-operatively. Error bars represent SEM.
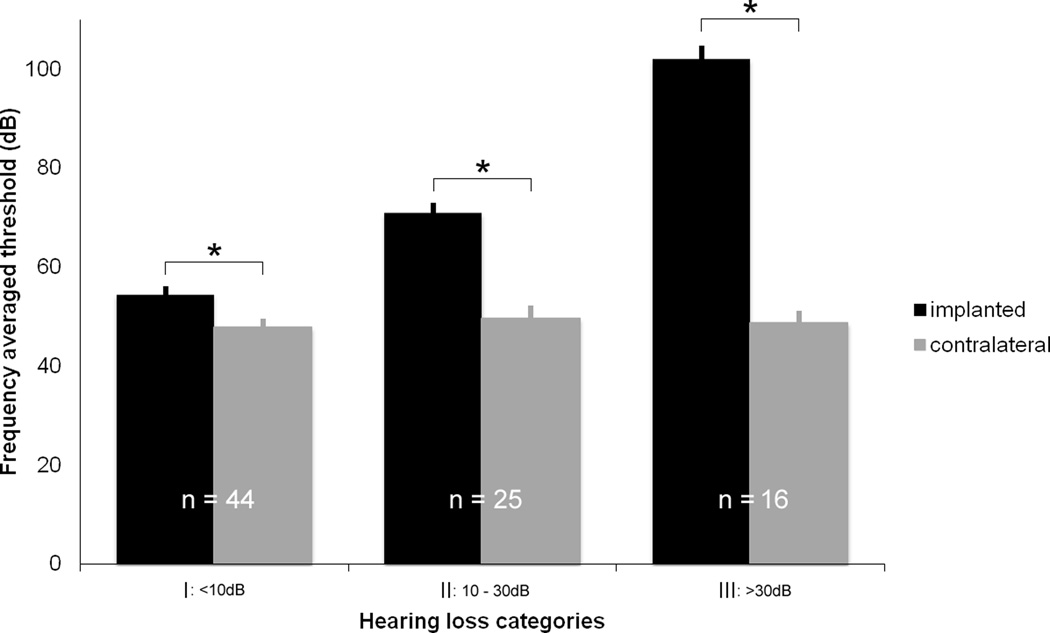
Figure 6 illustrates the changes in PTA thresholds in the implanted and contralateral ears one-year post implantation. Rate of HL was disregarded here and subjects were instead divided into three groups based only on the severity of the observed long-term difference in HL between ears. Group I subjects N=44/85 (52%) had less than a 10 dB difference between the two ears. This difference between the two ears is significant (paired T-test, p < 0.01). 25 patients (29%) had a 10.1–30 dB shift greater than the contralateral (Group II). All of these subjects retained sufficient hearing to use acoustic plus electric hearing. mean = 71 +/− 2 dB). 16 patients (19%) had a relative threshold shift greater than 30 dB (Group III), which, for 9 participants, led to an absolute threshold greater than 100 dB (mean = 102 +/− 3 dB). Thus only 9 of 85 patients (10.6%) experienced a post-implantation threshold shift that effectively eliminated residual hearing in the implanted ear. Figure 6 also demonstrates that the HL in the unimplanted ear was roughly equivalent for patients across each of these three categories.
Figure 6.
Contralateral HL is equivalent irrespective of ipsilateral loss. All patients were stratified by total threshold increase in implanted ears at 12 months post-implantation. Absolute threshold is represented for both implanted and contralateral ears (error bars = SEM). Only 19% had loss >30 dB (Group III). Contralateral threshold shift was equivalent across groups indicating that HL in Group III was not due to progression of premorbid cochlear pathology.
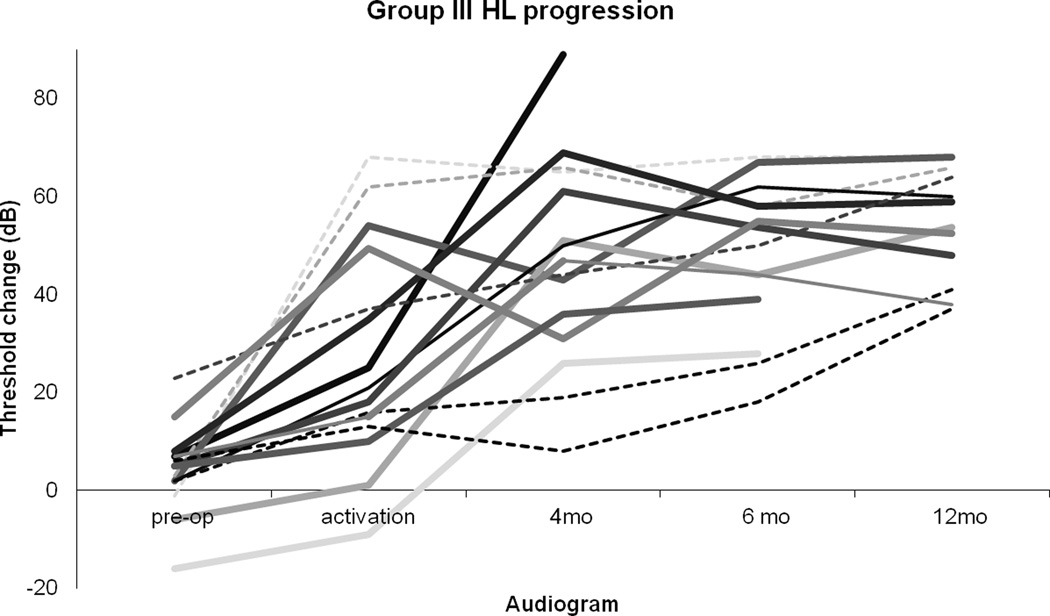
When the time course of HL for Group III patients is plotted individually (Figure 7), it is apparent that threshold increased markedly and/or continued to increase after activation for many patients (bold lines), including some who went on to have the worst hearing outcomes. As opposed to Groups I and II, only a minority of patients in Group III experienced the greatest rate of hearing loss between surgery and activation (dashed lines). For example, of the nine patients that lost all residual hearing, four lost most of their hearing after activation.
Figure 7.
HL increased after activation for many of those with the worst loss. Serial thresholds for Group III (threshold change >30 dB) patients depicted. Bold lines represent patients with marked HL after activation. Dashed lines represent patients for whom HL rate peaked between implantation and activation.
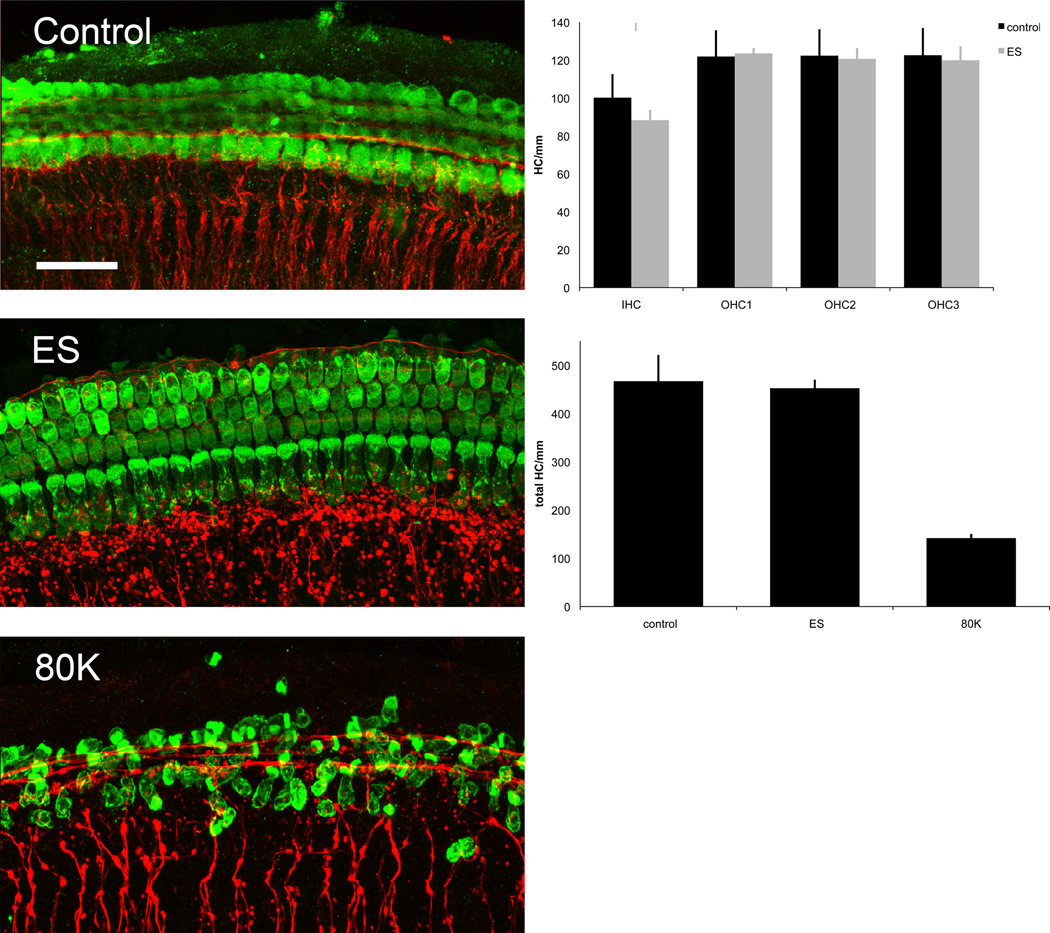
The post-activation HL in the five subjects with high charge levels, the small subset of subjects from the FDA trial that had post activation acceleration of HL and the association of high charge with high post-activation rates of HL led us to explore the possibility that high levels of ES may damage susceptible cochlear structures using an in vitro model. To assess which cochlear elements might be most susceptible to high levels of ES we used an organotypic cochlear preparation. Organotypic cultures exposed to high voltage (10V) ES (n = 14) were examined in comparison to negative (n = 19) and positive (n = 12) controls. As noted above, the overall charge delivered with this system was roughly an order of magnitude greater (10×) than that delivered by CIs. Preliminary experiments indicated that 3V and 5V amplitude stimulation at 10 Hz for three hours did not cause a discernable loss of hair cells or neuron/neurite damage. There were no significant differences in IHC or OHC density after ES (10V) compared with the negative control (Figure 8). Further the organ of Corti and most HCs did not exhibit any morphological changes following ES. On the other hand, chronic membrane depolarization with elevated extracellular potassium (80K) significantly reduced IHC and OHC density and altered hair cell morphology compared to explants maintained in control and ES conditions (control 467 HC/mm, ES 452 HC/mm, 80K 142 HC/mm, p<0.001).
Figure 8.
Hair cells counts are unaffected by high voltage ES while depolarizing media is toxic to hair cells. Depicted are IHC and OHC rows in Control, ES and 80K conditions. Scale bar = 20 µm. No significant differences were found in HC density of any subtype between ES and control conditions. Top chart, error bars = SEM. Decreased HC density was seen in organotypic cultures plated in depolarizing (80K) media when compared to both ES and control conditions (p<0.001). All HC types were summed here due to the inability to distinguish row in the 80K condition. Bottom chart, error bars = SEM.
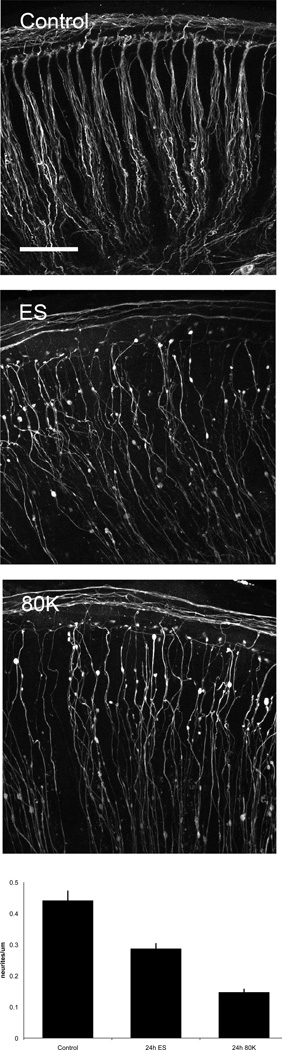
In contrast to the minimal effects seen in the organ of Corti, acute ES resulted in significant damage to the peripheral afferent processes (neurites) emanating from SGNs to innervate HCs. Both ES and 80K conditions caused loss of contact with the sensory epithelium and blebbing of the distal terminals with loss of a significant number of neurites (Figure 9). When quantified, neurite density (number neurites/m) was decreased significantly in the ES (65% of control, p<0.001) as well as 80K condition (33% of control, p<0.001).
Figure 9.
Distal peripheral process density decreases with both high voltage ES and depolarizing media. Depicted are peripheral processes in Control, ES and 80K conditions. Scale bar = 20 µm. The number of neurites/m differed significantly between all conditions (control, ES, 80K, p<0.001). Neurite density in ES and 80K conditions were 65 and 33% of control, respectively. Error bars = SEM. Qualitatively, axonal blebbing and loss of normal neurite bundle morphology increased along a similar gradient.
Discussion
Immediate minor increase in acoustic hearing thresholds following hearing preservation cochlear implantation averaged 12.2dB and is likely related to insertional trauma or change in fluid dynamics related to placement of the implant in the scala tympani. This high rate of hearing preservation is consistent with previous reports12,14. For 80% of patients, the rate of HL slowed significantly or even reversed after this initial post-operative period. Another group of subjects (20%) experienced greater than 5dB/month of threshold change following activation of the electrical processing and application of acoustic amplification. The fact that five patients demonstrated similar change following high intensity stimulation suggests that high-amplitude EAS might negatively impact acoustic hearing. This association is most marked with subject A1, who had minimal change in hearing threshold for over 6 months until charge levels were increased. Thus, the possibility that high-intensity EAS damages residual acoustic hearing was first suggested by the five patients reviewed, and is presented here for the first time.
Critical information can be inferred from examining the natural history of post-implantation HL using serial audiometry. First, only 20% of all Hybrid S8 recipients experienced acceleration of HL after activation. Thus, while mild post-implantation HL is common, late ipsilateral HL infrequently occurs. Furthermore, this HL is not limited to the higher frequencies, which would suggest basal cochlear pathology that might be attributed to a delayed local response to the implanted electrode array. Finally, the time course is delayed and occurs after electrode activation. In some cases the HL follows previous increases in charge stimulation level, thus it is less likely to be due to some immediate change in cochlear physiology after implantation, and more likely to be related to combined EAS itself. Of note, many of the subjects that experience severe changes in hearing are unaware of a dramatic threshold shift at the time of hearing loss. Thus, these events are less likely to be secondary to an abrupt event and more likely to represent exacerbation of an ongoing process. Indeed, the need for high amplitude ES based on psychophysical testing may indicate a priori susceptibility. Other mechanisms, such as electrode migration, intracochlear fibrosis or perilymphatic fistula, may also contribute, fully or in part, to the accelerated hearing loss following activation. However, the patients did not complain of other symptoms typical of perilymphatic fistula such as dizziness and there was no change in the number of functional electrodes.
This association between high ES charge and late ipsilateral hearing loss is confirmed by our odds ratio analysis which indicated that high charge is associated with an increased likelihood of developing significant post-activation hearing loss (odds ratio = 16). If we extrapolate from the FDA S8 trial and accept the prevalence of late acceleration of hearing loss in hearing preservation implantees as 20%, we can estimate the relative risk of late hearing loss as roughly three times higher in those exposed to high charge relative to those who are not exposed. This univariate analysis may be confounded by additional variables, such as implant type, materials or patient health status – which our dataset does not address17. Finally, intracochlear pathologies (e.g. fibrosis) that might necessitate higher charge to reach stimulable neural elements of the cochlea cannot be excluded based on these data18. Nonetheless, the strength of the association discovered here is sufficient to provide a basis for further study and should be considered by CI audiologists when programming hearing preservation cochlear implantees. Unfortunately, initial HL in the first month after implantation is identical between patients that go on to have rapid loss of hearing after activation and those that do not – making it difficult to predict which patients are most susceptible (Figure 5) to the late HL.
The effect of chronic ES on intact cochlear structures has been well documented in hearing cats showing HC loss in close proximity to the implant with no additional damage to the sensory epithelium secondary to ES19,20. Using TEM, Dodson et al showed that round window ES in hearing guinea pigs causes damage to efferent synapses on the OHCs but largely spares HCs and afferent neurons21. More recently, Coco et al showed no damage to functional HCs and small but significant rescue of SGNs apical to an electrode array after implantation and chronic ES in partially deafened cats22. Post-mortem human histopathological studies likewise show preservation of IHCs and OHCs apical to the array with no deleterious effect on the density of SGNs23. Guinea pig models of hearing preservation implantation have shown that acoustic hearing loss correlates with scala tympani fibrosis24. Our preliminary evidence from exposure of organotypic cultures to high voltage ES points towards a primary effect at the level of the afferent nerve terminal and/or synapse with little effect on HCs.
Animal studies indicate that excitotoxic trauma to the cochlea primarily damages afferent nerve terminals and afferent/IHC synapses25. Puel et al examined the effects of high intensity (120+ dB) noise in hearing guinea pigs demonstrating that in addition to expected mechanical damage to OHCs, afferent peripheral nerve terminals were swollen and/or disrupted26. This effect was attributed to glutamate excitotoxicity, the predominant afferent neurotransmitter, in that infusion of a glutamate antagonist mitigated nerve terminal, but not OHC damage. They hypothesized that excess synaptic glutamate prolongs depolarization causing osmotic imbalance, nerve terminal swelling and possibly even membrane disruption. More recently Wang and Green showed a significant loss of post-synaptic densities after exposure of organotypic cultures to glutamate agonists27. Once again, HCs and presynaptic ribbons were largely spared. Thus, afferent nerve terminals appear particularly sensitive to excitotoxicity. Furthermore there is evidence to suggest that even physiologic levels of glutamatergic synaptic activity may lead to peripheral neurite damage when neurons are subjected to an inflammatory environment and TNFα in particular28, 29.
The damage to SGN neurites following high voltage ES of cochleotypic cultures is consistent with the hypothesis that higher levels of ES damage afferent nerve terminals while largely sparing HCs. The stimulator used in these studies allows for control of voltage, but not current, output. It is not possible to replicate the stimulation parameters used in human subject to those used in vitro due vast differences in tissue and media impedances among other factors. Further, the exact current levels that the target cells are exposed to in in vivo or in vitro preparations remain unknown. Therefore, we titrated the voltage level to the point where we could first see an effect on the cytoarchitecture of our organotypic cultures. Damage was first observed at 10V, a voltage which produces approximately an order of magnitude greater charge/phase than levels used clinically in CI patients. Lower voltages caused no discernable effect. Further the stimulation level used in these experiments was not generally cytotoxic as hair cells, neurons, and other cells did not die over the time-course of observation. The only identifiable damage was degeneration of the peripheral afferent fibers, a finding similar to excitotoxic damage caused to brief exposures to loud noise in animal models and to glutamate agonists applied to cochlear explants like those used in this study25,26,27,30.
Potential mechanisms contributing to the deleterious effects of ES on afferent nerve terminals and synapses include the electrophonic response, antidromic propagation of action potentials31 and/or exacerbation of damage from acoustic noise trauma. These mechanisms may interact or combine in the context of resolving post-surgical inflammation to become even more excitotoxic. Electrophonic responses represent an increase in OHC motility induced by ES that in turn may increase basilar membrane/IHC responsiveness32. Electrophysiologic animal studies provide evidence that electrophonic responses contribute to EAS responses33. Interactions between acoustic and ES are nonlinear in that neuronal spike rate will asymptote at the pulse rate of the electrical stimulus34. In most cases, increase in ES rate or level results in increased masking of the acoustic response in a predictable fashion, likely related to electrically evoked refractoriness, limiting the likelihood of “overdriving” the nerve. Furthermore a gradient of interaction exists such that acoustic stimuli of low frequency and high amplitude are less masked35. However, Miller et al report that in rare instances (4% of studied fibers), ES responses are “enhanced” by acoustic stimuli such that simultaneous presentation increases neuron spike activity above ES alone in a nonlinear fashion34. Stronks et al similarly cite the electrophonic response to explain nonlinear and delayed characteristics of combined electric/acoustic masking. Antidromic stimulation of SGNs may interact with glutamatergic depolarization from electrophonic stimulation to enhance excitotoxic damage. Likewise, noise damage may also be more damaging in this milieu of post-surgical inflammation and high levels of ES.
Intra-operative steroid delivery to the inner ear has been shown to almost completely ameliorate immediate post-implantation hearing loss in animals36–39. In human CI recipients, intra-operative methylprednisolone infused into the middle ear affords a small but significant amount of protection for residual hearing40. Proposed mechanisms include inhibition of inflammatory cascades by blocking TNFα and downstream apoptotic pathways41, 42. Again, it cannot be determined whether the delayed acceleration of hearing loss seen in a minority of patients (20%) in this series represents a continuation of a post-surgical inflammatory or fibrotic process that may be mitigated by glucocorticoids or, as we propose, an excitotoxic process.
These potential mechanisms of HL are unique to EAS and require further study, particularly in that they suggest diverse therapeutic modalities. Prevention efforts in this patient population to date have focused on limiting surgical insertion trauma11 or its downstream inflammatory effects, primarily using glucocorticoids37–40. Our data suggest that limiting high intensity acoustic and electrical signals may also play a role in protecting ipsilateral hearing. Steroid infusion into the inner ear at the time of implantation may mitigate the inflammatory milieu in which excitotoxicity occurs but ultimately may not address the underlying pathological process. Finally, these patient data are valuable in that they invoke the need to model and unravel the physiologic and anatomic effects of combined acoustic and electrical stimuli in cochleae with substantial low frequency residual hearing.
Conclusions
Ipsilateral loss of acoustic hearing after hearing preservation cochlear implantation may limit benefit from combined modes of hearing. We show that in the few patients that lose significant acoustic hearing over a month after surgery, high charge has a moderate and statistically significant association with late hearing loss. Early evidence from organotypic culture points to the primary afferent nerve terminal/synapse as a likely site of damage. These data suggest that limiting exposure to potentially excitotoxic stimuli (e.g. loud noise, high pulse amplitude and pulse width) may be critical for protection of low frequency hearing in susceptible individuals. This information also informs and facilitates development of appropriate cochleotypic and animal models to further characterize and potentially ameliorate this phenomenon.
Acknowledgements
We would like to gratefully acknowledge the guidance and assistance of our collaborators and colleagues in the Cochlear Implant Center at the University of Iowa Hospitals and Clinics. In particular we would like to thank Jake Oleson and Camille Dunn for their invaluable input and support.
Funding: This research was supported in part by research grant 2P50DC000242-26A1 from the National Institutes on Deafness and Other Communication Disorders, National Institutes of Health; grant RR00059 from the General Clinical Research Centers Program, Division of Research Resources, National Institutes of Health; the Lions Clubs International Foundation; and the Iowa Lions Foundation.
Footnotes
Conflicts of Interest: Author BJG holds a patent on the Hybrid Cochlear Implant. No royalties are received. Additionally he is a consultant for both Advanced Bionics and Cochlear Corporation.
References
- 1.Gantz BJ, Turner CW. Combining acoustic and electrical hearing. Laryngoscope. 2003 Oct;113(10):1726–1730. doi: 10.1097/00005537-200310000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Gantz BJ, Hansen MR, Turner CW, Oleson JJ, Reiss LA, Parkinson AJ. Hybrid 10 clinical trial: preliminary results. Audiol Neurootol. 2009;14(Suppl 1):32–38. doi: 10.1159/000206493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dorman MF, Gifford RH, Spahr AJ, McKarns SA. The benefits of combining acoustic and electric stimulation for the recognition of speech, voice and melodies. Audiol Neurootol. 2008;13(2):105–112. doi: 10.1159/000111782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turner CW, Gantz BJ, Vidal C, Behrens A, Henry BA. Speech recognition in noise for cochlear implant listeners: benefits of residual acoustic hearing. J Acoust Soc. Am. 2004 Apr;115(4):1729–1735. doi: 10.1121/1.1687425. [DOI] [PubMed] [Google Scholar]
- 5.Gfeller KE, Olszewski C, Turner C, Gantz B, Oleson J. Music perception with cochlear implants and residual hearing. Audiol Neurootol. 2006;11(Suppl 1):12–15. doi: 10.1159/000095608. [DOI] [PubMed] [Google Scholar]
- 6.Lenarz T, Stöver T, Buechner A, Lesinski-Schiedat A, Patrick J, Pesch J. Hearing conservation surgery using the Hybrid-L electrode. Results from the first clinical trial at the Medical University of Hannover. Audiol Neurootol. 2009;14(Suppl 1):22–31. doi: 10.1159/000206492. [DOI] [PubMed] [Google Scholar]
- 7.Brockmeier SJ, Peterreins M, Lorens A, Vermeire K, Helbig S, Anderson I, Skarzynski H, Van de Heyning P, Gstoettner W, Kiefer J. Music perception in electric acoustic stimulation users as assessed by the Mu.S.I.C. test. Adv Otorhinolaryngol. 2010;67:70–80. doi: 10.1159/000262598. [DOI] [PubMed] [Google Scholar]
- 8.Dunn CC, Perreau A, Gantz B, Tyler RS. Benefits of localization and speech perception with multiple noise sources in listeners with a short-electrode cochlear implant. J Am Acad Audiol. 2010 Jan;21(1):44–51. doi: 10.3766/jaaa.21.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turner CW, Reiss LA, Gantz BJ. Combined acoustic and electric hearing: preserving residual acoustic hearing. Hear Res. 2008 Aug;242(1–2):164–171. doi: 10.1016/j.heares.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golub JS, Won JH, Drennan WR, Worman TD, Rubinstein JT. Spectral and temporal measures in hybrid cochlear implant users: on the mechanism of electroacoustic hearing benefits. Otol Neurotol. 2012 Feb;33(2):147–153. doi: 10.1097/MAO.0b013e318241b6d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roland PS, Wright CG. Surgical aspects of cochlear implantation: mechanisms of insertional trauma. Adv Otorhinolaryngol. 2006;64:11–30. doi: 10.1159/000094642. [DOI] [PubMed] [Google Scholar]
- 12.Gifford RH, Dorman MF, Spahr AJ, Bacon SP, Skarzynski H, Lorens A. Hearing preservation surgery: psychophysical estimates of cochlear damage in recipients of a short electrode array. J Acoust Soc Am. 2008 Oct;124(4):2164–2173. doi: 10.1121/1.2967842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Podskarbi-Fayette R, Pilka A, Skarzynski H. Electric stimulation complements functional residual hearing in partial deafness. Acta Otolaryngol. 2010 Jan 28; doi: 10.3109/00016480903567189. [DOI] [PubMed] [Google Scholar]
- 14.Woodson EA, Reiss LA, Turner CW, Gfeller K, Gantz BJ. The Hybrid cochlear implant: a review. Adv Otorhinolaryngol. 2010;67:125–134. doi: 10.1159/000262604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeon EJ, Xu N, Xu L, Hansen MR. Influence of central glia on spiral ganglion neuron growth. Neuroscience. 2011;177:321–334. doi: 10.1016/j.neuroscience.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roehm PC, Xu N, Woodson EA, Green SH, Hansen MR. Membrane depolarization inhibits spiral ganglion neurite growth via activation of multiple types of voltage sensitive calcium channels and calpain. Mol Cell Neurosci. 2008;37(2):376–387. doi: 10.1016/j.mcn.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kopelovich JC, Reiss LAJ, Oleson JJ, Lundt ES, Gantz BJ, Hansen MR. Risk factors for loss of ipsilateral residual hearing after hearing preservation cochlear implantation. Otol Neurotol. doi: 10.1097/MAO.0000000000000389. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jia H, Wang J, François F, Uziel A, Puel JL, Venail F. Molecular and cellular mechanisms of loss of residual hearing after cochlear implantation. Ann Otol Rhinol Laryngol. 2013 Jan;122(1):33–39. doi: 10.1177/000348941312200107. [DOI] [PubMed] [Google Scholar]
- 19.Shepherd RK, Clark GM, Black RC. Chronic electrical stimulation of the auditory nerve in cats. Physiological and histopathological results. Acta Otolaryngol Suppl. 1983;399:19–31. doi: 10.3109/00016488309105589. [DOI] [PubMed] [Google Scholar]
- 20.Ni D, Shepherd RK, Seldon HL, Xu SA, Clark GM, Millard RE. Cochlear pathology following chronic electrical stimulation of the auditory nerve. I: Normal hearing kittens. Hear Res. 1992 Sep;62(1):63–81. doi: 10.1016/0378-5955(92)90203-y. [DOI] [PubMed] [Google Scholar]
- 21.Dodson HC, Walliker JR, Bannister LH, Douek EE, Fourcin AJ. Structural effects of short term and chronic extracochlear electrical stimulation on the guinea pig spiral organ. Hear Res. 1987 Nov;31(1):65–78. doi: 10.1016/0378-5955(87)90214-0. [DOI] [PubMed] [Google Scholar]
- 22.Coco A, Epp SB, Fallon JB, Xu J, Millard RE, Shepherd RK. Does cochlear implantation and electrical stimulation affect residual hair cells and spiral ganglion neurons? Hear Res. 2007 Mar;225(1–2):60–70. doi: 10.1016/j.heares.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linthicum FH, Jr, Fayad J, Otto SR, Galey FR, House WF. Cochlear implant histopathology. Am J Otol. 1991 Jul;12(4):245–311. [PubMed] [Google Scholar]
- 24.O'Leary SJ, Monksfield P, Kel G, Connolly T, Souter MA, Chang A, Marovic P, O'Leary JS, Richardson R, Eastwood H. Relations between cochlear histopathology and hearing loss in experimental cochlear implantation. Hear Res. 2013 Apr;298:27–35. doi: 10.1016/j.heares.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Pujol R, Puel JL. Excitotoxicity, synaptic repair, and functional recovery in the mammalian cochlea: a review of recent findings. Ann N Y Acad Sci. 1999 Nov 28;884:249–254. doi: 10.1111/j.1749-6632.1999.tb08646.x. [DOI] [PubMed] [Google Scholar]
- 26.Puel JL, Ruel J, Gervais d'Aldin C, Pujol R. Excitotoxicity and repair of cochlear synapses after noise-trauma induced hearing loss. Neuroreport. 1998 Jun 22;9(9):2109–2114. doi: 10.1097/00001756-199806220-00037. [DOI] [PubMed] [Google Scholar]
- 27.Wang Q, Green SH. Functional role of neurotrophin-3 in synapse regeneration by spiral ganglion neurons on inner hair cells after excitotoxic trauma in vitro. J Neurosci. 2011 May 25;31(21):7938–7949. doi: 10.1523/JNEUROSCI.1434-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellizzi MJ, Lu SM, Masliah E, Gelbard HA. Synaptic activity becomes excitotoxic in neurons exposed to elevated levels of platelet-activating factor. J Clin Invest. 2005 Nov;115(11):3185–3192. doi: 10.1172/JCI25444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han P, Whelan PJ. Tumor necrosis factor alpha enhances glutamatergic transmission onto spinal motoneurons. J Neurotrauma. 2010 Jan;27(1):287–292. doi: 10.1089/neu.2009.1016. [DOI] [PubMed] [Google Scholar]
- 30.Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after "temporary" noise-induced hearing loss. J Neurosci. 2009 Nov 11;29(45):14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Briaire JJ, Frijns JH. Unraveling the electrically evoked compound action potential. Hear Res. 2005 Jul;205(1–2):143–156. doi: 10.1016/j.heares.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 32.Xue S, Mountain DC, Hubbard AE. Electrically evoked basilar membrane motion. J Acoust Soc Am. 1995 May;97(5 Pt 1):3030–3041. doi: 10.1121/1.413103. [DOI] [PubMed] [Google Scholar]
- 33.Miller CA, Abbas PJ, Robinson BK, Nourski KV, Zhang F, Jeng FC. Electrical excitation of the acoustically sensitive auditory nerve: single-fiber responses to electric pulse trains. J Assoc Res Otolaryngol. 2006 Sep;7(3):195–210. doi: 10.1007/s10162-006-0036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller CA, Abbas PJ, Robinson BK, Nourski KV, Zhang F, Jeng FC. Auditory nerve fiber responses to combined acoustic and electric stimulation. J Assoc Res Otolaryngol. 2009 Sep;10(3):425–445. doi: 10.1007/s10162-008-0154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stronks HC, Versnel H, Prijs VF, Klis SF. Suppression of the acoustically evoked auditory-nerve response by electrical stimulation in the cochlea of the guinea pig. Hear Res. 2010 Jan;259(1–2):64–74. doi: 10.1016/j.heares.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Eshraghi AA, Adil E, He J, Graves R, Balkany TJ, Van De Water TR. Local dexamethasone therapy conserves hearing in an animal model of electrode insertion trauma-induced hearing loss. Otol Neurotol. 2007 Sep;28(6):842–849. doi: 10.1097/mao.0b013e31805778fc. [DOI] [PubMed] [Google Scholar]
- 37.Vivero RJ, Joseph DE, Angeli S, He J, Chen S, Eshraghi AA, Balkany TJ, Van de Water TR. Dexamethasone base conserves hearing from electrode trauma-induced hearing loss. Laryngoscope. 2008 Nov;118(11):2028–2035. doi: 10.1097/MLG.0b013e31818173ec. [DOI] [PubMed] [Google Scholar]
- 38.James DP, Eastwood H, Richardson RT, O'Leary SJ. Effects of round window dexamethasone on residual hearing in a Guinea pig model of cochlear implantation. Audiol Neurootol. 2008;13(2):86–96. doi: 10.1159/000111780. [DOI] [PubMed] [Google Scholar]
- 39.Eastwood H, Chang A, Kel G, Sly D, Richardson R, O'Leary SJ. Round window delivery of dexamethasone ameliorates local and remote hearing loss produced by cochlear implantation into the second turn of the guinea pig cochlea. Hear Res. 2010 Jun 14;265(1–2):25–29. doi: 10.1016/j.heares.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 40.Rajan GP, Kuthubutheen J, Hedne N, Krishnaswamy J. The role of preoperative,intratympanic glucocorticoids for hearing preservation in cochlear implantation: A prospective clinical study. Laryngoscope. 2012 Jan;122(1):190–195. doi: 10.1002/lary.22142. [DOI] [PubMed] [Google Scholar]
- 41.Haake SM, Dinh CT, Chen S, Eshraghi AA, Van De Water TR. Dexamethasone protects auditory hair cells against TNFalpha-initiated apoptosis via activation of PI3K/Akt and NFkappaB signaling. Hear Res. 2009 Sep;255(1–2):22–32. doi: 10.1016/j.heares.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 42.Dinh CT, Haake S, Chen S, Hoang K, Nong E, Eshraghi AA, Balkany TJ, Van De Water TR. Dexamethasone protects organ of corti explants against tumor necrosis factor-alpha-induced loss of auditory hair cells and alters the expression levels of apoptosis-related genes. Neuroscience. 2008 Nov 19;157(2):405–413. doi: 10.1016/j.neuroscience.2008.09.012. [DOI] [PubMed] [Google Scholar]