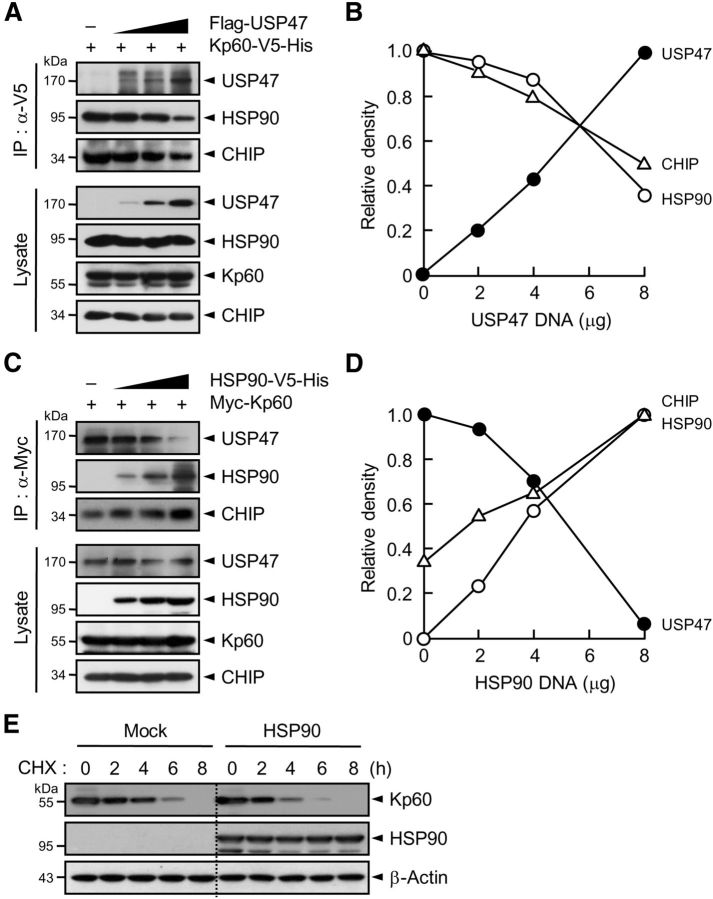
Figure 5.
USP47 competes with HSP90 for binding to katanin-p60. A, Kp60–V5-His was expressed in HEK293T cells with and without increasing amounts of Flag-USP47. Cell lysates were subjected to immunoprecipitation with anti-V5 antibody followed by immunoblot with anti-HSP90, anti-V5, or anti-Flag antibody. B, The intensity of each protein band in A was scanned using a densitometer and was quantified using ImageJ software. The intensity of HSP90 or CHIP seen with an empty vector was expressed as 1.0, and the others were as its relative values. For USP47, the intensity seen upon transfection of 8 μg of USP47 cDNA was expressed as 1.0, and the others were expressed as its relative values. C, Myc-Kp60 was expressed with and without increasing amounts of HSP90–V5-His. Cell lysates were immunoprecipitated with anti-Myc antibody followed by immunoblot with anti-Myc, anti-V5, or anti-CHIP antibody. D, The intensity of each protein band in C was quantified as in B. The intensity of USP47 seen with an empty vector was expressed as 1.0, and the others were expressed as its relative values. For CHIP and HSP90, the intensity seen upon transfection of 8 μg of HSP90 cDNA was expressed as 1.0, and the others were expressed as its relative values. E, Neuro2A cells were transfected with pcDNA (Mock) or pcDNA-HSP90. After incubation for 24 h, cells were treated with cycloheximide. Note that in A and C, cells were incubated with 10 μm MG132 for 4 h before the preparation of cell lysates.