Fig. 4.
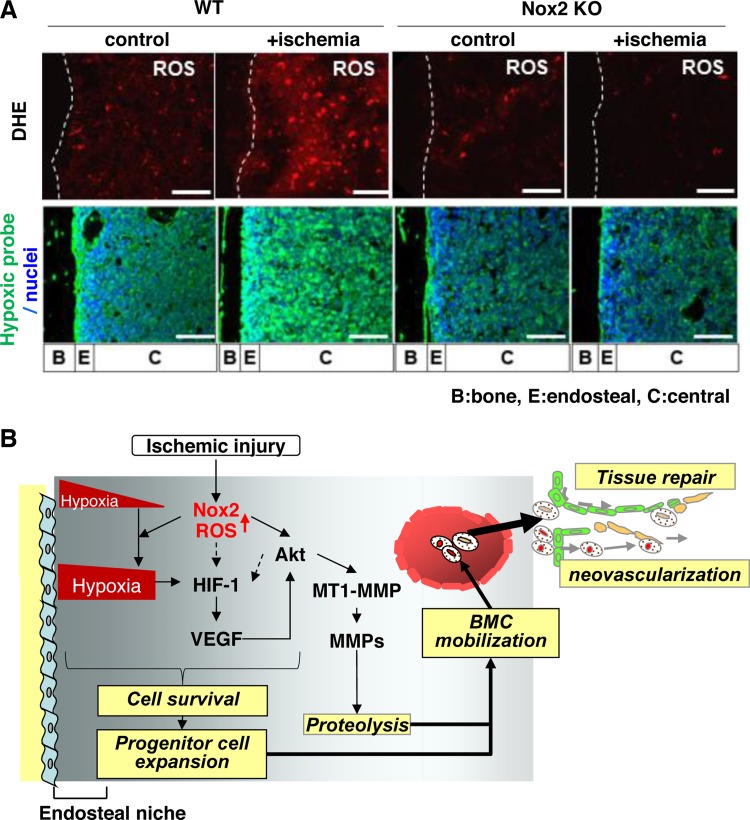
A: Nox2 is involved in superoxide (O2·−) production in bone marrow (BM) in situ in response to hindlimb ischemia. Representative images of O2·− production in BM of femur from wild-type (WT) or Nox2 knockout (KO) mice subjected to hindlimb ischemia [+ischemia (day 3)] or without hindlimb ischemia (control). To detect O2·− in situ, dihydroethidium (DHE) was injected 60 min before tissue harvest. Long bone surface is shown by dotted lines. Bottom: hindlimb ischemia increases hypoxic area in BM, in a Nox2-dependent manner. Mice were injected with pimonidazole 3 h before euthanization. Femur sections from mice subjected to hindlimb ischemia [+ischemia (day 3)] or without ischemia (control) were stained with anti-pimonidazole antibody (green) and 4′,6-diamidino-2-phenylindole (DAPI) to visualize nuclei (blue). Bars show 100 μm. Bone (B), endosteal (E) (defined as 50 μm apart from the bone surface), and central (C) regions are indicated. B: Nox2 regulates BM microenvironment involved in progenitor cell function and mobilization in response to ischemia. After ischemic injury, ROS production is increased in entire BM in a Nox2-dependent manner, which is required for increasing hypoxic niche and its downstream HIF-1α and VEGF expression in BM. Nox2-derived ROS also increase Akt phosphorylation and its downstream membrane type1-matrix metalloproteinase (MT1-MMP) expression and MMP-9 activity in BM. It is possible that ROS and Akt pathway are involved in HIF-1α expression through hypoxia-independent mechanism (dotted arrows). These Nox2-dependent alterations of BM microenvironment promote progenitor cell survival and expansion, thereby promoting their mobilization, leading to reparative neovascularization and tissue repair (modified from Ref. 88).