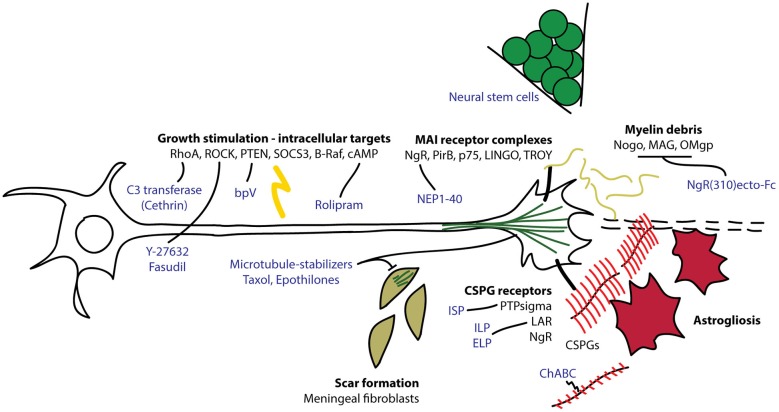
Figure 1.
Overview of intrinsic and extrinsic molecules involved in axon regeneration after CNS injury. Myelin debris from damaged oligodendrocytes and chondroitin sulfate proteoglycans (CSPGs) produced by reactive astrocytes interact with receptors on the growth cones to interfere with axon extension. Intracellular signaling molecules regulate the ability of the neuron to regenerate its damaged axon through the lesion environment. C3 transferase (Cethrin) inhibits RhoA to promote axon extension and cell survival. Fasudil and Y-27632 inhibit Rho kinase (ROCK) to promote axon extension. Bisperoxovanadium (bpV) inhibits phosphatase and tensin homolog (PTEN) to promote neuroprotection. Rolipram inhibits phosphodiesterase 4 (PDE4) to stabilize cAMP and promote axon extension. Microtubule stabilizers, taxol and epothilones, reduce fibrotic scarring and promote axon extension. Chondroitinase ABC (ChABC) digests CSPG glycosaminoglycan (GAG) chains (in red), relieving CSPG-dependent growth inhibition. Intracellular sigma peptide (ISP) inhibits protein tyrosine phosphatase sigma (PTPsigma) and intracellular/extracellular leukocyte common antigen related phosphatase (LAR) peptides (ILP/ELP) inhibit leukocyte antigen-related receptor (LAR) to neutralize the inhibitory effect of CSPGs and promote axon sprouting. NgR(310)ecto-Fc competes for binding to Nogo, myelin-associated glycoprotein (MAG) and Oligodendrocyte myelin glycoprotein (OMgp) to relieve myelin-dependent growth inhibition. Nogo extracellular peptide 1–40 (NEP1–40) competes for NgR binding to neutralize inhibitory signaling through NgR.