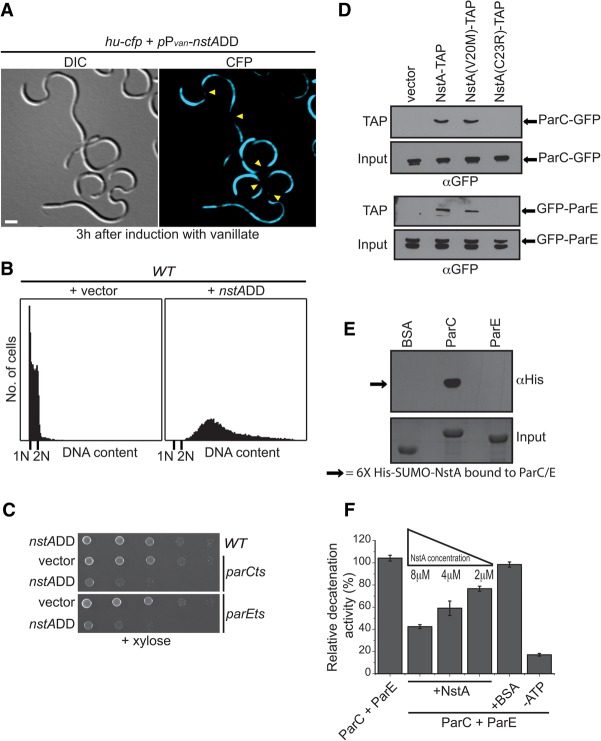
Figure 3.
NstA inhibits the activity of topo IV. (A) DIC and fluorescence microscopy images of wild-type cells overexpressing nstADD from the Pvan promoter on the high copy vector pMT335 and harboring the nonspecific chromosome-binding protein HU fused at the C terminus with the cyan fluorescent protein (CFP) expressed from the native chromosomal locus (hu::hu-cfp). The cells were induced with 0.5 mM vanillate for 3 h to induce the production of NstADD. Yellow arrowheads denote the chromosome-free regions. Bar, 2 μm. (B) Flow cytometry profiles to show the DNA content of wild-type (WT) cells overexpressing nstADD in comparison with cells harboring the empty vector alone. Overexpression of nstADD was done as described in A. (C) Growth of the wild-type cells and the temperature-sensitive parCts and parEts mutants in the presence and absence of nstADD. The expression of nstADD was from the chromosomal xylX locus (xylX::Pxyl-nstADD). Fivefold dilutions of the indicated strains were spotted onto medium containing 0.3% xylose. The cells were grown at permissive temperature. (D) Immunoblots of TAP samples of extracts from wild-type cells harboring parC-gfp (top panel) or gfp-parE (bottom panel) expressing NstA-TAP, NstA(V20M)-TAP, or NstA(C23R)-TAP from Pvan on pMT335. The extracts of parC-gfp or gfp-parE strains with the empty vector were used as a control. Anti-GFP (αGFP) was used for detection. (E) Far-Western analysis using purified ParC, ParE, and His6-SUMO-NstA. (Top panel) A blot containing 0.1 nM ParC, ParE, or BSA was incubated with 25 nM purified His6-SUMO-NstA and further probed with monoclonal hexa-Histidine antibody (αHis). (Bottom panel) A Coomassie brilliant blue-stained gel with 0.1 nM BSA, ParC, and ParE is shown as the input control. (F) Relative in vitro DNA decatenation activity of topo IV (ParC + ParE) in the presence of various concentrations of NstA. The reactions were carried out with either ParC and ParE alone or together with increasing concentrations of NstA as indicated. Reactions with BSA equivalent to 8 μM NstA or without ATP were used as controls. The data are the average of three independent experiments ±SE.