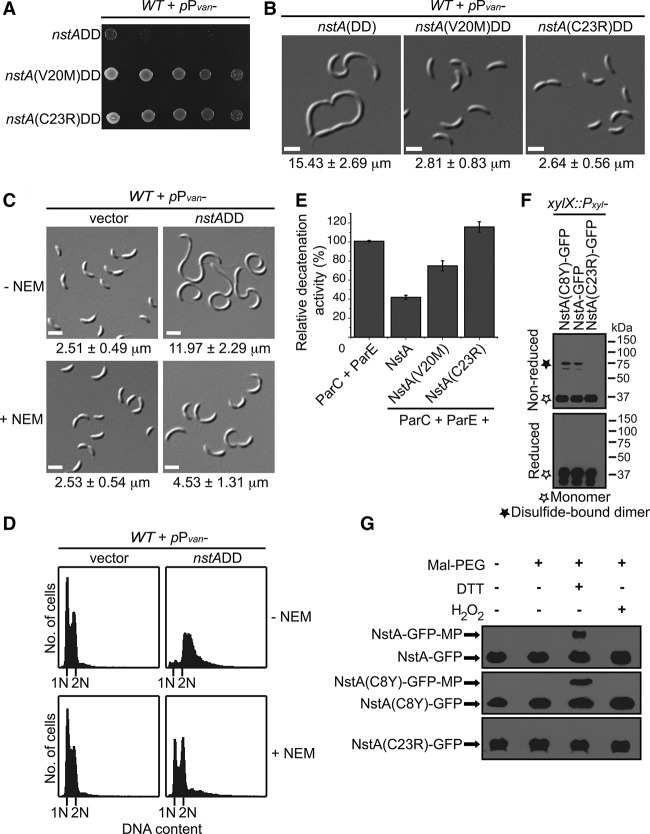
Figure 4.
Cysteine disulfide-dependent activity of NstA. (A) Growth of wild-type (WT) cells overexpressing nstADD, nstA(V20M)DD, and nstA(C23R)DD from Pvan on pMT335. Fivefold diluted cultures were spotted onto medium containing 0.5 mM vanillate. (B) DIC microscopy images of cells in A. (C) DIC microscopy images of wild-type cells overexpressing nstADD from Pvan on pMT335 in the presence or absence of N-ethylmaleimide (NEM). Cells were pretreated with 7.5 μM NEM for 2 h prior to the addition of 0.25 mM vanillate. (D) Flow cytometry profiles to show the DNA content of cells in C. (E) Relative in vitro DNA decatenation activity of topo IV (ParC + ParE) in the presence of purified NstA, NstA(V20M), and NstA(C23R). (F) Immunoblots of reducing and nonreducing SDS-PAGE of NstA-GFP, NstA(C8Y)-GFP, and NstA(C23R)-GFP expressed from the xylX locus on the chromosome. (G) Immunoblots of NEM and maleimide-PEG (MP) modifications. The samples were extracted in the presence of excess NEM that immediately blocks all free thiol groups. Subsequently, disulfide bridges were reduced by the addition of DTT, and the newly formed thiol groups from the reduction of the disulfide bonds by DTT were modified with MP (molecular weight 5 kDa). Increased-molecular-weight form [NstA-GFP-MP or NstA(C8Y)-GFP-MP] denotes the existence of disulfide bridges. No disulfide bridge was formed in NstA(C23R), leading to the absence of higher-molecular-weight forms. Mean cell size ± SD of at least 200 cells is given at the bottom of the images in B and C.