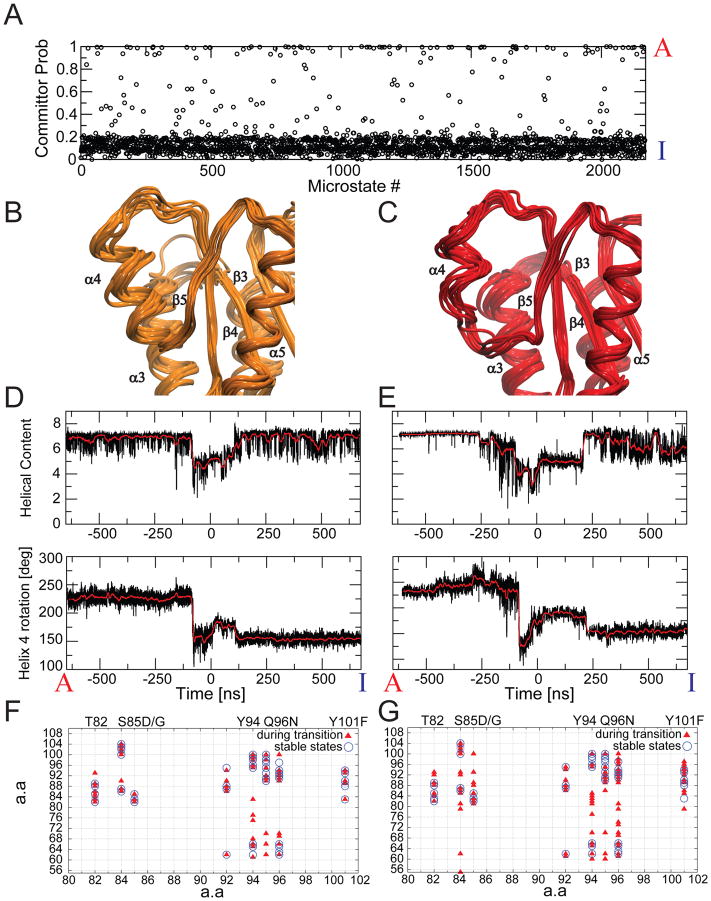
Fig. 3. Multiple pathways connect the inactive and active states.
A) Committor probabilities for each of the 2168 microstates in the MSM, defining the 5 most populated microstates belonging to the inactive and active macrostates as reactant and products, respectively. B,C) Superposition of states with committor probabilities close to 0.5 indicates qualitative differences, ranging from an intact helix 4 (B) to a partially unfolded helix 4 (C). D,E) Helical content and rotation angle of helix 4 during trajectories describing a full transition between active and inactive states. F,G) H-bonds involving polar side-chains in helix 4 during trajectories describing a full transition between active and inactive states via transition states with a nearly intact helix 4 (F), and with a partially unfolded helix 4 (G). H-bonds observed during the middle segment of the transition are shown as triangles, while H-bonds present in the two stable end states are represented as open circles. On the horizontal axis we marked relevant amino-acids including mutations which resulted in an increase in the activation barrier without affecting I and A 13.