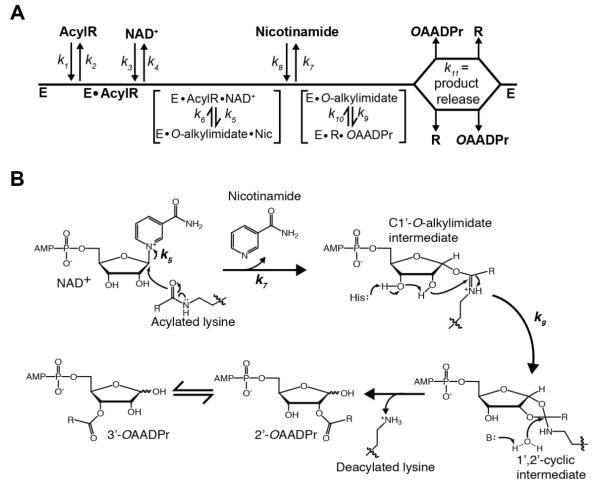
Figure 1. Proposed Sirtuin deacylation mechanism and kinetic scheme.
A, Sirtuin enzymes follow a sequential mechanism in which both acylated substrate (AcylR) (k1) and NAD+ (k3) bind prior to any catalytic step. SIRT1, SIRT2 and SIRT3 must bind AcylR prior to NAD+, while SIRT6 is the only mammalian Sirtuin capable of binding AcylR or NAD+ in random order. A ternary complex is formed, followed by nicotinamide formation (k5) and release (k7), and transfer of the acyl group from AcylR to ADP-ribose (k9). Deacylated substrate (R) and O-acyl-ADPr (OAADPr) are randomly released (k11). B, Proposed deacylation mechanism. Nucleophilic addition of the acyl oxygen on the 1′-carbon of the nicotinamide ribose forms the C1′-O-alkylimidate intermediate. A conserved histidine residue in the active site activates the 2′-hydroxyl group of NAD+ ribose. The activated hydroxyl attacks the O-alkylimidate carbon to afford the 1′2′-cyclic intermediate. A base activated water molecule attacks the cyclic intermediate resulting in the formation of deacylated lysine and OAADPr. Rate constants are indicated.