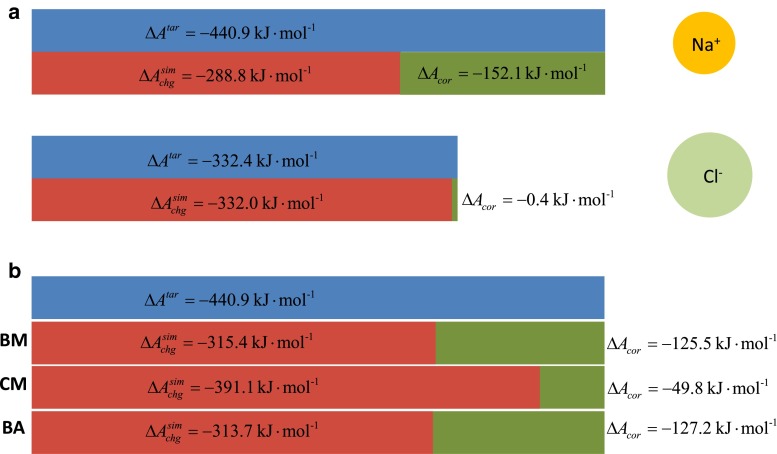
Fig. 1.
Effect of applying finite-size and approximate-electrostatics corrections [63, 64] to the charging free energies of cationic and anionic molecules, illustrated for the case of sodium and chloride ions with effective radii of [65] and 0.246 nm, respectively, and with Lennard-Jones parameters according to the GROMOS 54A8 force field [65, 66] in combination with the SPC water model [159]. a The charging free energies of the infinitely dilute ions in a macroscopic nonperiodic system with Coulombic electrostatic interactions are given by . For the spurious simulated situation of the BM scheme under periodic boundary conditions in a cubic computational box with nm, and nm, the charging free energies evaluate to . The correction terms, evaluated according to Ref. [64] are , , , and for the sodium ion and , , , and for the chloride ion, where the fitted functions described in Ref. [64] were used for and . b The magnitude of the overall correction term is reduced by and if an electrostatic potential restraint involving these two corrections is used. For the example of sodium ion hydration, these two quantities evaluate to [63, 64] or and or for the schemes with reaction-field correction (BM, BA) or the CM scheme, respectively. The correction term for the BM scheme thus amounts to . Its contributions (, , ) are reported in (a). For the CM scheme, has contributions from and ( and , respectively) and for the BA scheme, it has contributions from , and (, and respectively)