Table 4.
Cross-Coupling of Aryl Chlorides with Various Potassium Alkoxymethyltrifluoroboratesa
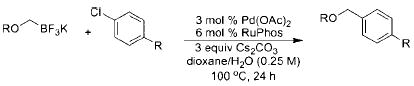
| |||||
|---|---|---|---|---|---|
| entry | nucleophile | product | isolated yield (%) | ||
| 1 |
|
2a |
|
R = CN 4a | 75 |
| OME4a’ | 77 | ||||
| 2 |
|
2b |
|
R = CN 4b | 72 |
| OME4b’ | 74 | ||||
| 3 |
|
2c |
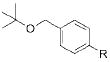
|
R = CN 4c | 67 |
| 4 |
|
2d |
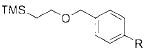
|
R = CN 4d | 80 |
| 5 |
|
2f |
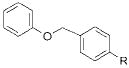
|
R = CN 4f | 48 |
| 6 |
|
2g |
|
R = CN 4g | 67 |
| OME4g’ | 60 | ||||
All reactions were carried out using 0.5mmol of aryl chloride and 0.55 mmol of alkoxymethyltrifluoroborate.
