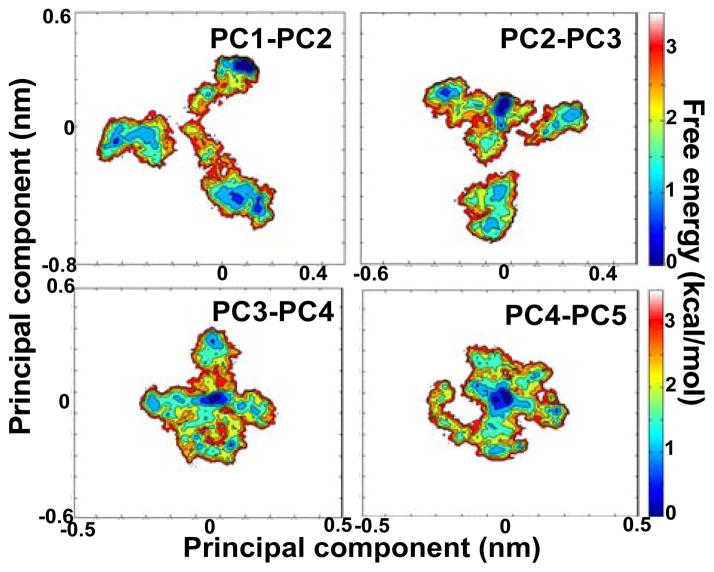
Fig. 2. Time-dependent structural fluctuations of Noxa.
(A) Evolution of the secondary structure of Noxa in the 2.5-μs trajectory. Secondary structure is colored as α-helix (pink), 310-helix (blue), β-strand (yellow) turn (cyan), and coil (white). (B) Snapshots illustrating the key structural features of Noxa as determined by 5-D dPCA clustering analysis. Each cluster is indicated in Roman numerals; the location of each cluster in the trajectory is shown with keys above panel A. The structure of Noxa is represented in ribbons and colored based on secondary structure, and N- and C-termini are shown as blue and red spheres, respectively.