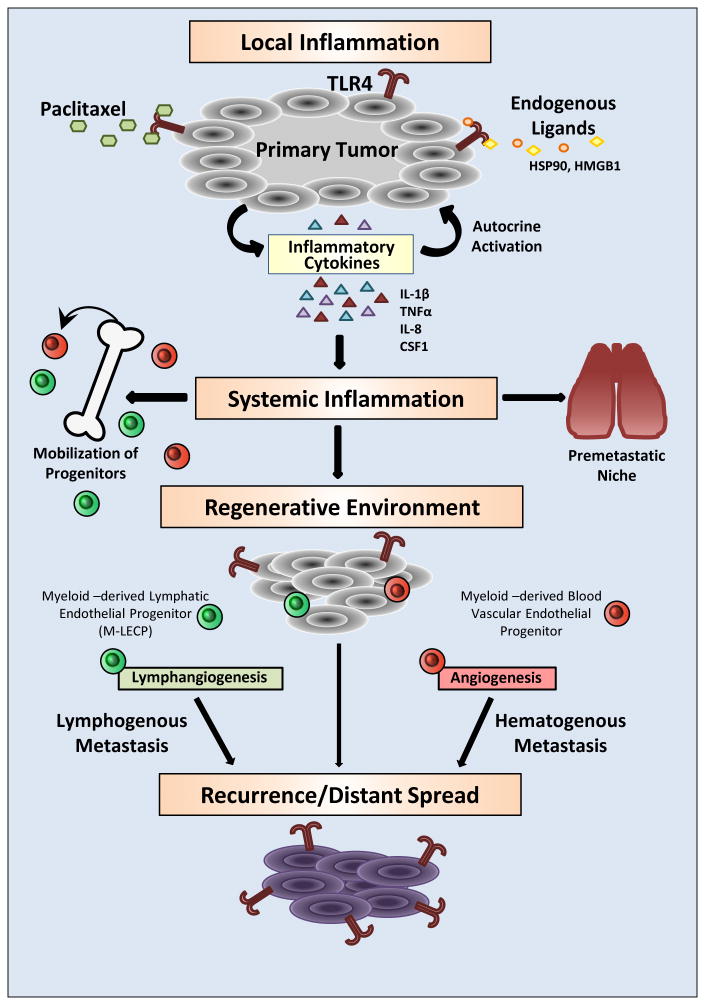
Figure 1. Prometastatic consequences of TLR4 activation in cancer cells driven by chemotherapy.
TLR4 expressed in tumor cells can be activated by a chemotherapeutic drug, paclitaxel, and numerous endogenous ligands including HSP90 and HMGB1. TLR4-activated NF-κB signaling leads to upregulation of metalloproteinases and inflammatory cytokines that enhance tumor motility and local inflammation. This proinflammatory shift protects tumor cells from the toxicity of anticancer drugs and endows them with better capacity to migrate out of the primary site. Excessive cytokines released to the blood generate systemic inflammation that causes expansion of bone marrow progenitors and their mobilization to the tumor. Blood-circulating progenitors also lodge in the lungs where they generate a highly receptive environment for passing tumor cells. Myeloid-derived blood vascular and lymphatic endothelial progenitors (M-LECP) create a regenerative environment typified by accelerated formation of new blood and lymphatic vessels. Increased migratory and survival potentials of tumor cells, coupled with increased blood and lymphatic vessel densities, results in local recurrence as well as lymphatic and distant metastasis. Systemic dissemination is also supported by generation of the premetastatic niche in lungs and other organs. Cumulatively, these events lead to multifocal disease largely resistant to the tumoricidal effects of cytotoxic therapies.