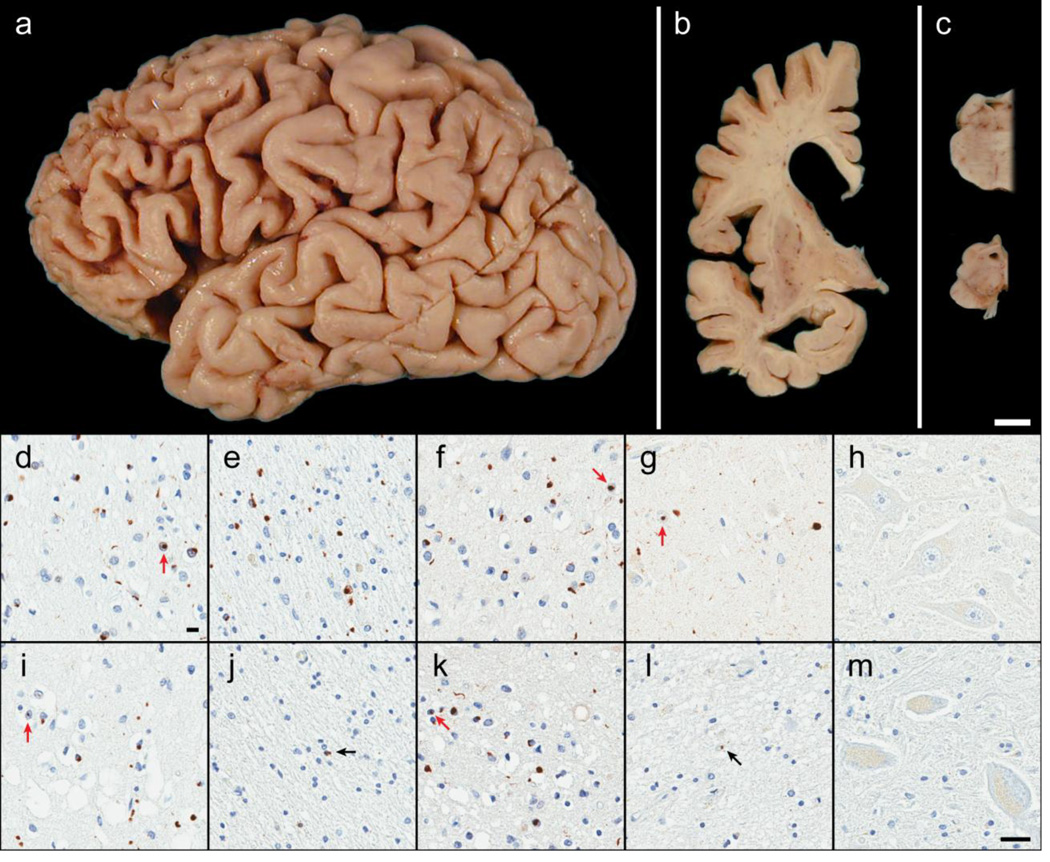
Figure 4. Neuropathology of OPTN and TBK1 double mutant carriers.
Case B exhibited focal cortical atrophy of the frontal lobe (a), ventricular enlargement of the frontal and temporal horns (b), atrophy or ‘flattening’ of the caudate (b), and loss of pigmentation in the substantia nigra (c) consistent with frontotemporal lobar degeneration. Microscopically, case B had abundant neuronal cytoplasmic inclusions, neuronal intranuclear inclusions (red arrows), glial cytoplasmic inclusions, and dystrophic neurites immunoreactive for p62/sequestosome-1 (d and e) as well as TDP-43 (f and g). These lesions, along with neuronal loss and superficial cortical spongiosis, were found in the gray matter of the frontal cortex (layer II; d and f) and, surprisingly, the underlying white matter of the frontal cortex (e). Neuronal loss and fine TDP-43-immunoreactive neurites in the CA1 of the hippocampus was indicative of hippocampal sclerosis (g). No pathology was found in motor neurons (hypoglossal nucleus) including with immunohistochemistry for optineurin protein (h). Case A had similar pathology in the gray matter of the frontal cortex (i and k) with p62 (i) and TDP-43 (k) immunohistochemistry in addition to pronounced superficial cortical spongiosis. Only rare immunoreactive glial cytoplasmic inclusions (black arrows) were observed the cortical white matter (p62; j) and in the hippocampal CA1 (TDP-43; l). Despite the paucity of hippocampal TDP-43 pathology, case A was also consistent with hippocampal sclerosis due to the vacuolation of the neuropil and severe neuronal loss. Again, no pathology was observed in the motor neurons (optineurin immunohistochemistry of the trigeminal motor nucleus; m). [white bar = 1 cm (a–c), black bar = 25 µm (d–m)]