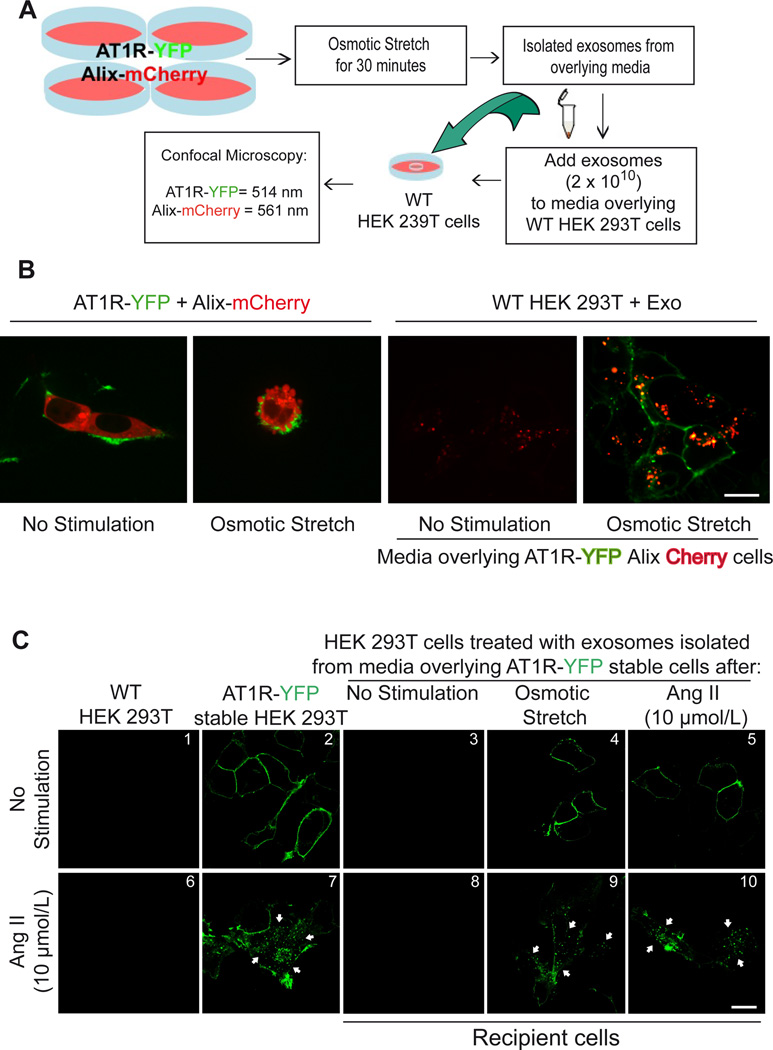
Figure 2.
HEK 293T cells respond to osmotic stretch and Angiotensin II by secreting AT1R enriched vesicles. A) Diagram of method used to show GPCR transfer by exosomes. B) confocal microscopy analysis of HEK 293T cells expressing AT1R-YFP and Alix-mCherry under basal conditions (panel I). 30 minutes of osmotic stress cells there is budding on the surface of cells and Alix (mCherry) and AT1R (YFP) enriched vesicles appear on the surface (panel II). Wild type HEK 293T cells 12 hours after being exposed to exosomes derived from not stimulated media with low transfer of Alix-Cherry protein (panel III) while hypotonic-stimulated conditioned media (panel IV) shows transfer of Alix-mCherry to the inside of cells and AT1R-YFP to the plasma membrane. C) Exosomes collected from overlying media of cells expressing AT1R-YFP were transferred to recipient cells and tested for their ability to internalize after agonist stimulation. Confocal microscopy analysis show AT1R-YFP localized to the plasma membrane in cells stably expressing AT1R-YFP (panel 2), and in recipient HEK293 cells after incubation with exosomes derived from osmotic stretch and AngII stimulation (panels 4 and 5). No GFP fluorescence was observed in recipient cells when incubated with exosomes isolated from unstimulated AT1R-GFP stable cells (panel 3). Stimulation of the transferred receptors with 10 µmol/L AngII for 15 min induced robust AT1R internalization (panels 9 and 10, white arrows). B,C (Scale bar 15 µm), N=4 independent experiments, representative images shown. The cell confluence (~70%) for all confocal experiments was checked in bright field using lower magnification microscope.