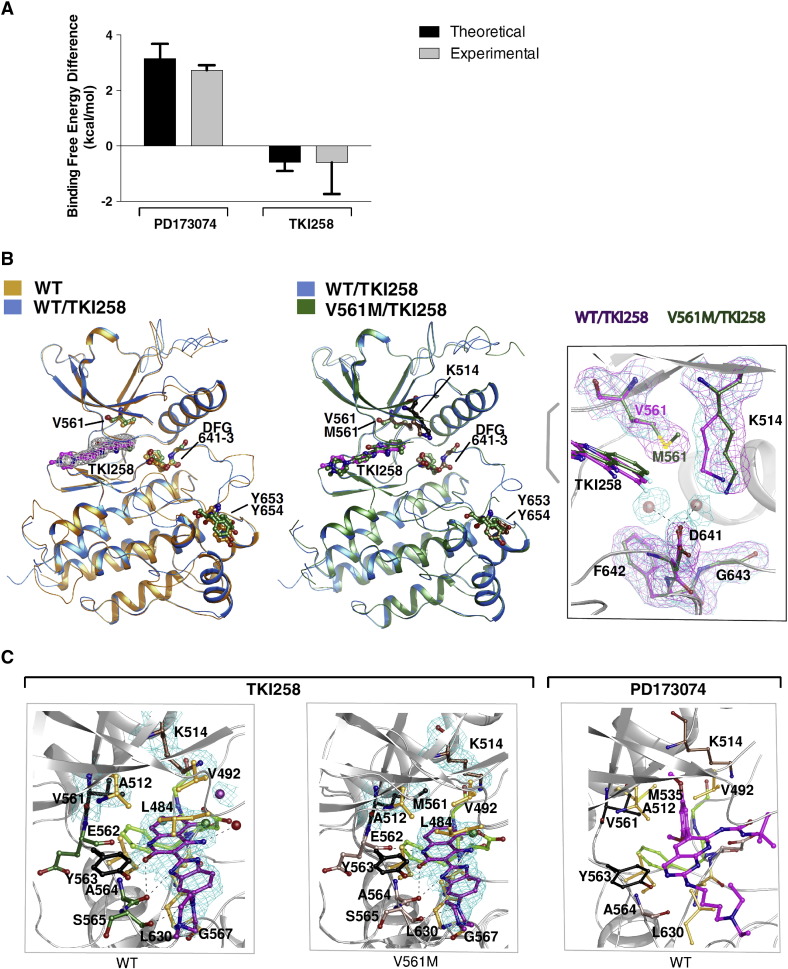
Fig. 2.
Binding of FGFR-inhibitors to wild type (WT) and V561M FGFR1 KD.
A. Histogram comparing the theoretical (generated through molecular dynamic simulations) and the experimental (generated through ITC) difference in free energy of the indicated inhibitors binding to FGFR1WT and FGFRV561M. See also Supplemental Table 3.
B. Superposition of apo WT (orange) and WT FGFR1 KD bound to TKI258 (marine) (left panel) and superposition of TKI258-bound WT (marine) and TKI258-bound V561M FGFR1 KD (forest green) (middle panel) are shown as ribbon representations. Electron density map (2Fo-Fc) contoured at 1.0 σ for TKI258 is shown for the WT (left panel). TKI258 bound to WT FGFR1 KD and V561M FGFR1 KD are coloured in magenta and forest green respectively. Several key residues, including DFG motif, gatekeeper residue and Y 653,654 are indicated. The right panel features superposition of TKI258 bound to WT FGFR1 KD and FGFR1 KD V561M showing the gatekeeper residue V561 and M561, and K514 displacement in FGFR1 KD V561M structure. Electron density map (2Fo-Fc) contoured at 1.0 σ is shown for the gatekeepr, DFG motif and Lys514 residues. The solvent molecules in WT FGFR1 KD at the active site are shown as red spheres along with electron density map (2Fo-Fc) controured at 1.0 σ.
C. Ball-and-stick representation of TKI258 bound WT FGFR1 KD and FGFR1 KD V561M is shown in the left and middle panel, respectively. Key residues are shown and labelled. Electron density map (2Fo-Fc) contoured at 1.0 σ is shown for TKI258, gatekeeper residue V/M561 and K514. Solvent molecule interacting with TKI258 is shown as red sphere. Hydrogen bonds are shown as black dashed lines. Fluoride atom is coloured in green. Ball-and-stick representation of WT FGFR1 KD bound PD173074 (PDB: 2FGI) and its interacting residues are shown in the right panel. In all panels DFG motif is coloured in lime, Y563, V/M561 in black, residues involved in hydrogen bonding interactions are coloured in salmon or green and residues involved in hydrophobic interaction and van der Waals contacts are in orange.