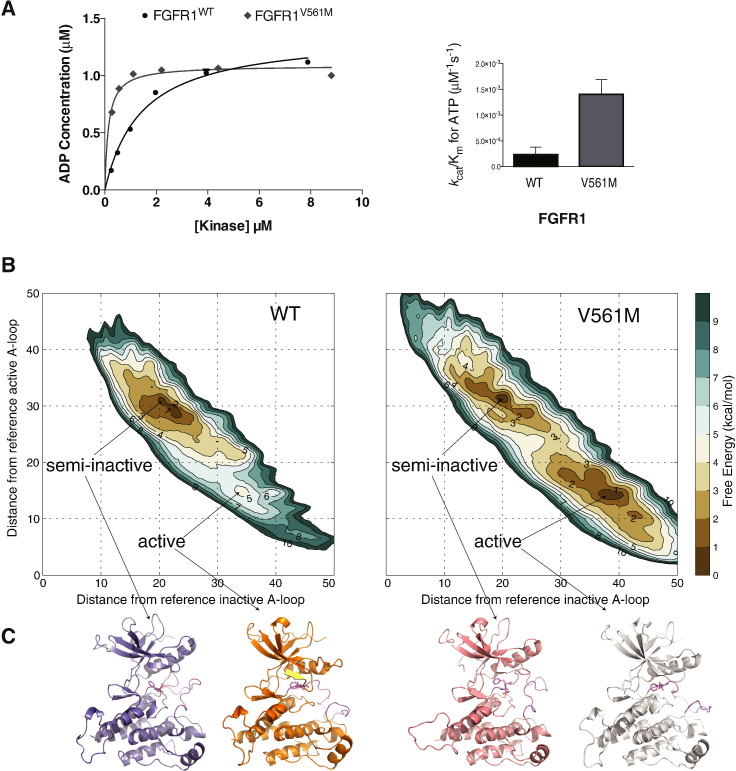
Fig. 3.
Impact of the gatekeeper substitution V561M on the kinase activity and overall structural changes of FGFR1 KD.
A. Left panel: comparison of the activity of FGFR1WT and FGFRV561M produced using the ADP-Glo Assay. Increasing concentrations of kinase were incubated for 60 min and the level of ADP produced analysed. Data were fit with a hyperbolic model (for presentation purposes only) using Graphpad prism. Each data point was produced in triplicate and the standard errors are indicated. Right panel: comparison of the enzyme efficiency (kcat/Km) of FGFR1WT and FGFRV561M. Parameters were generated through Michaelis–Menten kinetic experiments and analysed using Graphpad Prism software. See also Supplemental Tables S1 and S2.
B. The free energy surfaces of the activation of WT-FGFR and V561M-FGFR are shown as a function of the distance from the reference inactive A-loop conformation (CV1) and to the distance from the reference inactive A-loop conformation (CV2). The contour lines are drawn every 1 kcal/mol.
C. Ribbon representations of the proposed structures of FGFR1 WT and V561M coming from the full atomistic modelling molecular dynamics approach in the semi-inactive and active forms.