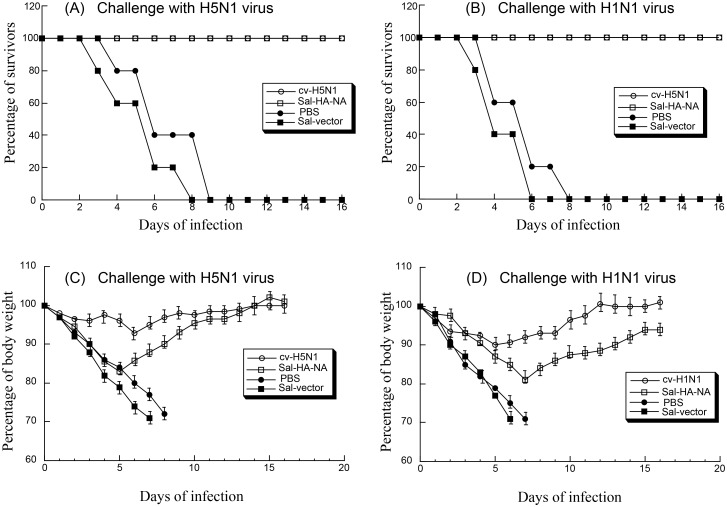
Fig 6. Immune protection of mice from lethal H5N1 (A and C) and H1N1 viral challenge (B and D).
Groups of mice (n = 5) were intragastrically immunized three times at day 0, 14, and 28 with PBS only, the commercial H5N1 (cv-H5N1) and H1N1 vaccines (cv-H1N1), and Salmonella SL368 carrying the empty vector pVAX1 (Sal-vector) and constructs p5HA and p5NA (Sal-HA-NA), and then challenged intranasally with 20 LD50 of lethal H5N1 (A/Viet Nam/1194R) and H1N1 (A/Puerto Rico/8/34) viruses at two weeks after final immunization (i.e. at 42 days post initial immunization). Mice were monitored for survival (A and B) and weight loss (C and D) throughout a 16 day observation period. The results are presented in terms of percent survival and percent of body weight (at the beginning of the trial) respectively. Humane (non-lethal) endpoints were used during the survival experiments and mice were euthanized with overdose inhalational CO2 when they exhibited weight loss of more than 30%, lethargy, ruffled hair coat, or hunched posture.