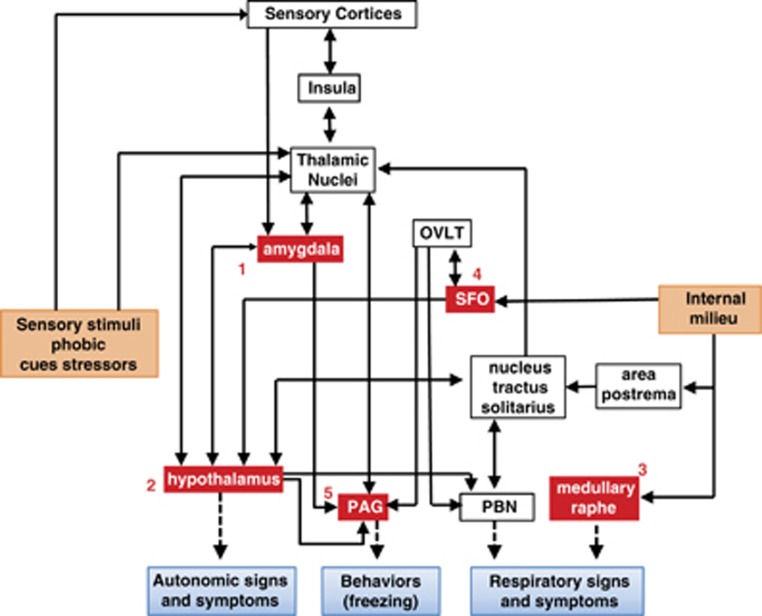
Figure 2.
Localization of chemosensory targets and regional circuits contributing to genesis and expression of panic. 1: acid sensing ion channels (ASICs) in the amygdala, 2: orexin neurons in the hypothalamus, 3: serotonergic neurons in the medullary raphe, 4: T-cell death-associated gene-8 receptor in the subfornical organ (SFO), 5: hypoxia-sensitive chemosensory neurons in the periaqueductal gray (PAG). Regions such as the SFO and medullary raphe can directly detect pH fluctuations in the internal milieu, while the hypothalamus, amygdala and PAG in addition to their chemosensory potential also represent key nodes in the processing of external threats, and sensory stimuli. Uncued panic may arise due to homeostatic imbalance in pH in the brain and internal milieu. Acidosis ‘sensed' by chemosensory mechanisms may be translated to autonomic, behavioral and respiratory symptoms of a panic attack. The amygdala, PAG and the hypothalamus can regulate behavioral and autonomic symptoms of panic, whereas respiratory symptoms may be regulated by brain stem regions such as the medullary raphe and the parabrachial nucleus (PBN) via inputs from the hypothalamus and indirectly from the SFO through the organum vasculosum of the lamina terminalis (OVLT). Many of these structures via thalamic nuclei connect with the insula, a region relevant for interoceptive sensing and shown to be dysfunctional in PD. Cued panic attacks may be an outcome of sensory stimuli and phobic cues associated with previous attacks or stressors relayed via sensory cortices and thalamic nuclei to the amygdala and the hypothalamus. It is important to note the overlap and connectivity between pH chemosensory regions and exteroceptive threat processing areas suggesting that uncued and cued panic may recruit similar underlying circuitry depending on modality of the trigger leading to panic.