Abstract
Advances in HIV-1 vaccine clinical trials and preclinical research indicate that the virus envelope glycoproteins (Env) are likely to be an essential component of a prophylactic vaccine. Efficient antigen uptake and presentation by dendritic cells (DCs) is important for strong CD4+ T helper cell responses and the development of effective humoral immune responses. Here, we examined the capacity of distinct primary human DC subsets to internalise and present recombinant Env to CD4+ T cells. Consistent with their specific receptor expression, skin DCs bound and internalised Env via C-type lectin receptors (CLRs) while blood DC subsets, including CD1c+ myeloid DCs (MDCs), CD123+ plasmacytoid DCs (PDCs) and CD141+ DCs exhibited a restricted repertoire of CLRs and relied on CD4 for uptake of Env. Despite a generally poor capacity for antigen uptake compared to MDCs, the high expression of CD4 on PDCs allowed them to bind and internalise Env very efficiently. CD4-mediated uptake delivered Env to EEA1+ endosomes that progressed to Lamp1+ and MHC class II+ lysosomes where internalised Env was degraded rapidly. Finally, all three blood DC subsets were able to internalise an Env-CMV pp65 fusion protein via CD4 and stimulate pp65-specific CD4+ T cells. Thus, in the in vitro systems described here, CD4-mediated uptake of Env is a functional pathway leading to antigen presentation and this may therefore be a mechanism utilised by blood DCs, including PDCs, for generating immune responses to Env-based vaccines.
Keywords: plasmacytoid dendritic cells, myeloid, Langerhans cells, HIV-1, gp120, CD4, vaccine, antigen uptake, presentation
Introduction
Dendritic cells (DCs) are the most potent antigen presenting cells and essential for establishing effective adaptive immune responses. Their capacity for uptake and presentation of vaccine antigens is likely to dictate the quality and efficiency of the ensuing vaccine-induced response. A primary goal of a prophylactic HIV-1 vaccine is to elicit potent, broadly neutralising antibodies (nAbs) that provide the first line of defense against virus exposure. The HIV-1 envelope glycoproteins (Env) are the only surface-exposed, virally encoded proteins of the HIV-1 virion and as the sole target for nAbs are likely to be included in a vaccine (1, 2). One of the critical factors in the establishment of an effective humoral response is the provision of appropriate CD4+ T cell-mediated help. Thus, the efficiency with which DCs stimulate CD4+ T cells following vaccination is likely to influence the quality of a developing B cell response.
The diversity of DC subsets suggests they play distinct roles in stimulating innate and adaptive immune responses. In the event of an intramuscular vaccination, DCs are likely to infiltrate the muscle from the blood and potentially also the skin, and interact with vaccine antigens early in the immune response. Myeloid DCs (MDCs) found in the skin and blood have potent antigen uptake capacity (3) and express a variety of receptors including C-type lectin receptors (CLRs), which they employ for endocytic or phagocytic uptake of exogenous antigen. In contrast, plasmacytoid DCs (PDCs) have a more restricted capacity for uptake of soluble antigen (4–7). Different receptor expression and uptake pathways can lead to different intracellular routing and consequently a different outcome in the mode or efficiency of antigen presentation (5, 8–10). DC subsets also have intrinsic differences in their antigen processing capacity due to variations in the expression level of proteins involved in MHC processing (3, 11, 12). MDCs are generally considered more potent antigen presenting cells for T cell priming. The distinctly different PDCs play an important role in immediate anti-viral responses through their capacity to produce large amounts of type I interferons, which among a plethora of functions support B cell differentiation and Ab production (13–16). However, the contribution of human PDCs in presentation of exogenous antigen to naïve CD4+ T cells to prime adaptive immune responses is less well defined (5, 7, 17, 18).
In this study we examined key interactions between soluble HIV-1 Env gp120 of the YU2 strain with human skin and blood DC subsets to understand the fate of these interactions. We found that skin DC subsets utilised CLRs to bind and internalise Env very efficiently. Conversely, blood MDCs including the BDCA-3+ cross-presenting subset, and PDCs utilised the high affinity HIV-1 receptor, CD4, for Env uptake, despite expression of purported Env-binding CLRs. Strikingly, CD4-mediated uptake of Env by PDCs was at least as efficient as uptake by MDCs and led to compartmentalisation of Env in EEA1+ endosomes, and subsequently into Lamp1+ and MHC class II+ lysosomes in both subsets. Antigen uptake via CD4 resulted in antigen processing and presentation by all blood DCs, demonstrating a novel mechanism of uptake of Env for the stimulation of Env-specific CD4+ T cells.
Materials and Methods
Isolation of blood DCs
This study was approved by the Institutional Review Board of Ethics at the Karolinska Institute, Stockholm, Sweden. Purified populations of immature MDCs and PDCs were isolated from blood from apheresed donors by positive selection with microbeads as previously described (13, 14, 19, 20) and cultured overnight at 37°C in complete medium (R10; RPMI/10% FCS/100U penicillin/0.1mg streptomycin, Sigma Aldrich, St Louis, MO, USA) supplemented with 2ng/mL GM-CSF (PeproTech, Rocky Hill, NJ, USA) where noted, or 1ng/mL IL-3 (R&D Systems, Minneapolis, MN, USA) respectively. The purity of the isolated cells was routinely greater than 85%. BDCA-3+ DCs were identified among enriched monocyte populations (Rosettesep monocyte enrichment kit, StemCell Technologies, Vancouver, Canada) from buffy coats by flow cytometry as HLA-DRhi, lineage− (CD3/CD14/CD19/CD20) and CD141+. For antigen presentation experiments DCs were sorted to very high purity (>98%) by flow cytometry using a FACSAria (Live Dead/CD3/CD20/CD14−, HLA-DR+ and either CD123+ (PDCs), CD11c/CD1c+ (MDCs) or CD11c/CD141+ (BDCA-3+ DCs)).
Isolation of skin DCs
Skin samples were obtained from patients undergoing breast re-construction surgery (Karolinska University Hospital, Stockholm, Sweden) and primary DCs were isolated as previously published (21), with some modifications. Skin pieces were sliced into nets using a skin graft mesher (Zimmer Inc. Warsaw, IN, USA) then incubated in 2U/mL Dispase (Gibco, Grand Island, NY, USA) overnight at 4°C to separate the epidermis from the dermis. Each fraction was incubated in 2.5mg/mL Collagenase F (Sigma Aldrich) with 20U/mL DNase I (Roche, Mannheim, Germany) in a gently shaking incubator (70–80 rpm) at 37°C for 1h. Following digestion, the cell suspensions were collected and washed several times with RPMI/5U/mL DNase I and passed through a 40µm cell strainer. LCs were identified by flow cytometry as HLA-DR+CD1a+ whereas DDCs could be identified as HLA-DR+, CD1a+/CD14− or CD1a−/CD14+. All DC subsets were immature after isolation with low CD83 expression and intermediate CD86 expression.
Phenotypic characterisation of DCs
DC subsets were phenotyped using different combinations of Abs including: HLA-DR, CD11c, CD1c, CD1a, CD14, CD4, CD40, CD83, CD80, CD86, and MR (BD Biosciences, San José, CA, USA), Langerin (Beckman Coulter), DC-SIGN and DCIR (R&D Systems), CD123, BDCA-2 and BDCA-3 (Miltenyi Biotec, Bergisch Gladbach, Germany). Cells were acquired on a FACSCalibur flow cytometer (BD Biosciences) and data analysed using FlowJo (Treestar Inc. Ashland, OR, USA).
Env antigens
Wild type gp120 monomers (from here on referred to as Env) from the primary R5 isolate YU2 and a CD4 binding-defective variant (gp120-D368R; from here on referred to as Env-D368R) (22) were used. Fusion proteins between Env or Env-D368R and human cytomegalovirus pp65 (strain AD169, accession P06725) were constructed by generating cDNA clones encoding Env and pp65 in a single reading frame (Supp. Fig. 1). Influenza haemagglutinin (HA) from the A/Perth/16/2009(H3N2) strain was used (accession ACS71642.1). Plasmid generation and site-directed mutagenesis were carried out by Geneart (Life Technologies, Carlsbad, CA, USA). All proteins were expressed in serum-free medium by transient transfection of Freestyle 293-F cells (Life Technologies) and LPS-extracted as described previously (23–25). Proteins were labeled with AlexaFluor 488 (AF488) Protein Labeling Kit or FluoReporter FITC Protein Labeling Kit (Molecular Probes, Eugene, OR, USA) according to the manufacturer’s instructions.
Antigen uptake and blocking assays
DCs at 1×106/mL were pulsed with 10µg/mL AF488 conjugated HIV-1 Env Ags or Influenza HA for 90 min at 37°C. For blocking studies the cells were pre-incubated with 5mg/mL mannan (Sigma Aldrich) or 100µg/mL CD4 Ab (RPA-T4) for 15 min at 4°C or 37°C prior to addition of Ag. Cells were then washed and analysed by flow cytometry.
CD4 internalisation
Whole PBMCs (for T cells) or monocyte-enriched PBMCs (for DCs) were incubated with anti-CD4 mAb (Clone SK-3, BD Biosciences) at 4°C for 20 min or 10µg/mL Env-AF488 at 4°C for 45 min. Cells were then washed in cold PBS and cultured in cold R10 on ice or at 37°C for 0, 60 or 120 min. At each time point cells were washed twice in either cold PBS or acetic acid buffer (0.2M acetic acid, 0.15M NaCl, pH 3.0) to remove residual surface CD4 Ab or Env, and resuspended in PBS/10mM HEPES to neutralise the pH. Cells were then surface stained with HLA-DR, lineage markers and either CD1c, CD123 or CD141 to identify DC populations by FACS.
Env degradation assay
DCs were incubated with 10µg/mL FITC-conjugated HIV-1 Env Ags, or HA at 37°C for 90 min. Cells were then washed three times and resuspended in cold R10 and cultured at 4°C or 37°C for up to 24 h. Cells were washed once more and stained with DC markers for analysis by flow cytometry. To retard degradation of antigens, cells were pre-incubated with, and the experiment and washes carried out in, the presence of a protease inhibitor 1mM Leupeptin (Roche).
Confocal microscopy
Purified DC subsets were exposed to Env Ags for between 10–120 min at 37°C then washed, applied to glass adhesion slides (Marienfeld GmbH, Lauda-Königshofen, Germany), fixed in 4% paraformaldehyde for 20 min at 4°C and permeabilised with 0.05% saponin for 20 min. The slides were blocked with 10% normal donkey serum (Sigma) for 30 min at 37°C then stained for 45 min at 37°C with EEA1 (clone 14, BD Biosciences) or Lamp-1 (clone H5G11, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) diluted in 10% NDS, followed by AlexaFluor-555 conjugated donkey anti-mouse IgG for 30 min at 37°C. The cell surface was then stained with AlexaFluor-647-conjugated HLA-DR (Biolegend, San Diego, CA, USA) and coverslips mounted using Pro-Long Gold containing DAPI (Molecular Probes). Analysis was done with a Leica DMIRE2 microscope and Leica TCSSP2 confocal system (Leica Microsystems, Heidelberg GmbH, Mannheim, Germany). Colocalisation data were quantified using JaCOP (ImageJ (26)) and Manders’ M1 co-efficient for the percentage of Env overlapping with an endolysosomal marker is shown.
Antigen presentation assays
Blood DC subsets, sorted to high purity by flow cytometry from donors with detectable CMV pp65-specific CD4+ T cell recall responses, were pulsed at 4°C for 1 h with Env-pp65, Env-D368R-pp65 or pp65 (Miltenyi Biotec) using equivalent amounts of pp65 (5–10µg/mL). Alternatively, overlapping 15-mer CMV pp65 peptides were used (Proimmune). The cells were then washed and co-cultured overnight with autologous CD4+ T cells (isolated using Rosettesep CD4+ T cell enrichment kit, Stem Cell Technologies) at a DC:T cell ratio of 1:10 in the presence of 10µg/mL brefeldin A (Sigma). Cells were then washed and stained for DC and T cell surface markers and intracellular IFN-γ, TNF and IL-2 (BD Biosciences).
Statistical Analyses
One-way ANOVA was used to test for differences in the degree of uptake of wt and D368R Env in different DC subsets and Tukey’s post-hoc comparisons were then used to compare the groups. Paired, two-tailed Student’s t-tests were performed for comparisons of colocalisation and antigen presentation (Prism 6, GraphPad Software, La Jolla, CA, USA) * p≤0.05, **p≤0.01, ***p≤0.001, ****p≤0.0001.
Results
Primary human DC subsets express distinct repertoires of receptors capable of binding HIV-1 Env
To define the uptake pathways of soluble HIV-1 Env by different DC subsets, immature primary DCs isolated from skin or blood were compared for their expression of a range of known and putative Env-binding receptors. During HIV-1 infection, Env binds the primary receptor CD4 which triggers a conformational change that allows the virus to engage CCR5 or CXCR4, leading to viral fusion. CLRs can also bind HIV-1 through their interaction with high mannose oligosaccharides on Env (27, 28) but these interactions do not mediate viral fusion. Consistent with earlier reports, we found that the most well-recognised Env-binding CLRs, including Langerin, mannose receptor (MR) and DC-SIGN, were all expressed on skin DCs. Freshly isolated HLA-DR+ CD1ahi Langerhans cells (LCs) from epidermis showed a characteristically high expression of Langerin (Fig. 1). Conversely HLA-DR+ dermal DCs (DDCs) predominantly expressed MR and DC-SIGN (Fig. 1). Specifically, the CD1a+ DDC subset expressed MR and a small proportion expressed Langerin while CD14+ DDCs expressed DC-SIGN and low amounts of MR. DEC-205 and DCIR, more recently proposed to bind HIV-1 (29, 30), were also expressed on both LCs and DDCs, particularly the CD1a+ DDCs. Finally, we detected low levels of CD4 expression on skin DCs as reported previously (31–33).
Fig. 1. Primary human DC subsets express distinct repertoires of HIV-1 Env receptors.
Freshly isolated immature DC subsets from epidermis, dermis and blood were phenotyped for their expression of known and putative Env-binding receptors. LCs were HLA-DR+/CD1a+. DDCs were HLA-DR+ and could be further sub-divided into CD1a+ and − (CD14+) subsets but these were pooled for this study. Blood DC subsets include HLA-DR+/CD11chi/CD1c+ MDCs, HLA-DR+/CD11cint/CD141(BDCA-3)+ MDCs and HLA-DR+/CD123+ PDCs. Data are representative of 3–7 donors for each marker. Dark histograms represent unstained cells.
The blood DCs were divided into three distinct populations - CD11chi CD1c+ MDCs (referred to as MDCs), the rare CD11cint CD141 (BDCA-3)+ MDCs (referred to as BDCA-3+ DCs) and CD123+ PDCs. In contrast to the skin, blood DCs displayed high levels of CD4, in particular PDCs, but were largely devoid of DC-SIGN, MR and Langerin (Fig. 1). MDCs could be induced to express low levels of MR in the presence of GM-CSF (Supp. Fig. 1). All three blood DC subsets expressed DEC-205 and DCIR. PDCs exclusively expressed BDCA-2 which in one study was suggested to have binding affinity for Env (34). Our data are consistent with recent gene expression profiles published for these subsets (35).
DC subsets utilise distinct receptors for soluble Env uptake
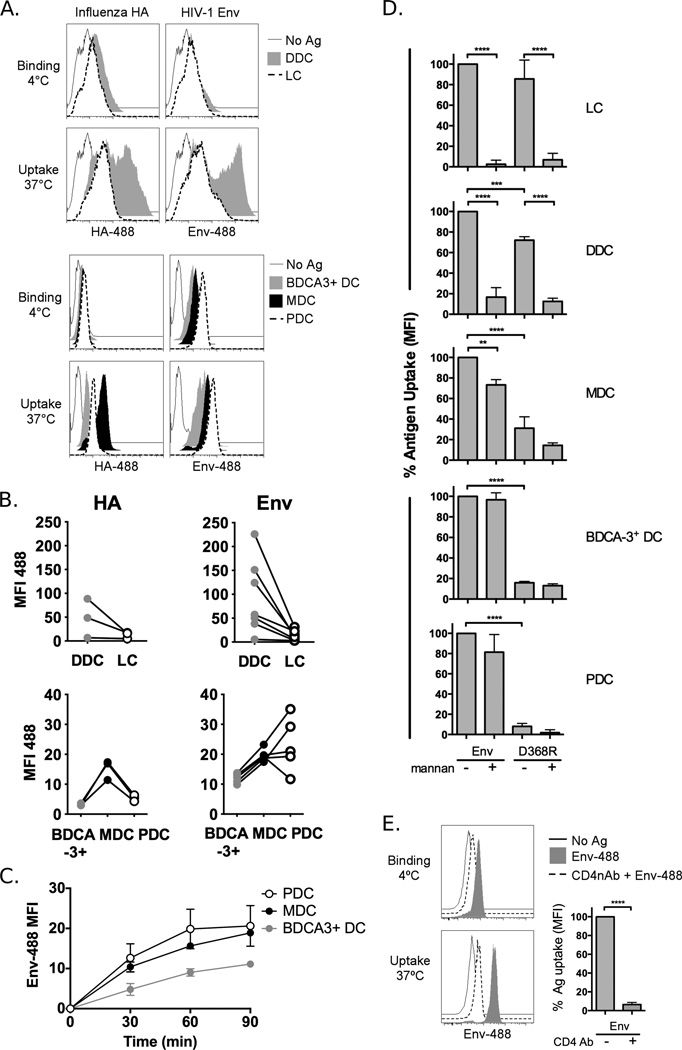
We next investigated the impact of this differential receptor expression on the capacity of the respective DC subsets to internalise a model Env immunogen, the YU2-based gp120 monomer. We used fluorophore-conjugated antigens to compare the degree of Env binding (at 4°C) and uptake (at 37°C) in the different DCs alongside another glycosylated viral surface antigen, influenza virus haemagglutinin (HA). The latter comprises the main-component of the seasonal influenza vaccine and was used here as a bench mark for antigen uptake of a clinically effective vaccine. Among the skin DCs, we found that DDCs were superior to LCs at both HA and Env uptake (Fig. 2a–b, upper panels). Of the blood DCs, MDCs are generally known as having superior antigen uptake capacity over PDCs. Consistent with this, MDCs displayed a higher capacity for HA uptake than PDCs but strikingly, donor-matched PDCs were as efficient, if not more efficient, than MDCs for Env binding and uptake (Fig. 2a–b, lower panels). BDCA-3+ DCs showed the lowest capacity for uptake of these two antigens (Fig. 2a–b, lower panels). Consistent with these data, the rates of internalisation of Env in MDCs and PDCs at 37°C were very similar, plateauing around 90 min (Fig. 2c). BDCA-3+ DCs tended to lag behind in their accumulation of Env, with significantly lower uptake at 60 and 90 min compared to PDCs (p<0.05).
Fig. 2. DC subsets utilise different receptors for Env uptake.
Skin and blood DCs were exposed to 10µg/mL AlexaFluor 488-labeled Influenza HA or HIV-1 Env for 90 min at 4°C to allow Ag binding or 37°C to allow Ag uptake and analysed by flow cytometry. A) Data from one representative skin donor and one representative blood donor is shown. B) The degree of Ag uptake (MFI 37°C – MFI 4°C) in each DC subset is shown for matched donors. C) The kinetics of Env uptake over the 90 min period are shown in blood DC subsets for 3 matched donors. D) DC subsets were incubated with Env or the CD4-binding defective Env-D368R in the absence or presence of mannan for 90 min at 4°C or 37°C or alternatively, E) PDCs were incubated with CD4 neutralising Ab (RPA-T4) prior to incubation with Env. The degree of uptake was calculated as for B) and normalised to Env alone. Data are the mean+SD for 3–5 donors. *p<0.05, **p<0.01, ***p<0.001
As Env and HA are both glycosylated, the uptake by DCs of both proteins may be mediated by CLRs. However, the discrepancy in uptake by PDCs indicates that Env can be internalised using alternative pathways. In order to dissect the contribution of CLRs versus CD4 for Env uptake by the different DC subsets we used mannan, a polymer of mannose that efficiently binds and blocks CLRs, as well as a gp120 variant containing a mutation in the CD4 binding site, which abolishes its ability to bind CD4 (Env-D368R) (Fig. 2d) (22). In both LCs and DDCs, Env uptake was largely inhibited by the presence of mannan while uptake of Env-D368R was only slightly reduced compared to wildtype (wt) Env. This indicates that these cells rely heavily on CLR-mediated uptake of Env. Conversely, blood DC subsets showed very poor uptake of Env-D368R compared to wt Env and exposure to mannan had, at most, a modest effect, indicating that Env uptake in these cells is predominantly dependent on CD4. This was confirmed in PDCs by blocking Env uptake with a CD4 neutralising Ab (Fig. 2e). However, due to the high expression of CD4 and internalisation of CD4 Ab that occurs in PDCs over time, and possibly also due to a difference in affinity, 10-fold excess of Ab was required for efficient blocking of Env. Thus the mutated version of Env, abolishing the CD4 binding site, was a more convenient and definitive control for our studies. While the reliance on CD4 was virtually complete for PDCs and BDCA-3+ DCs, the mannan block and uptake of Env-D368R were more pronounced in MDCs which may indicate low level usage of CLRs or non-specific antigen uptake mechanisms. This pattern of differential receptor usage between skin and blood DCs is consistent with previous findings (36) and despite expression of some proposed Env-binding CLRs in blood DCs, we found little evidence of their usage. This pattern of receptor usage was also confirmed using fibritin-stabilised, soluble gp140 (gp140-F) trimers (37) (Supp. Fig. 2).
CD4 mediates internalisation of Env in blood DCs
The primary function of CD4 is to act as a co-receptor during T cell receptor (TCR) communication with an antigen presenting cell. CD4 helps stabilise the immune synapse by binding directly to MHC class II and also amplifies the signal generated by the TCR. Since blood DCs were largely dependent on CD4 binding for uptake of Env, we next determined whether CD4 itself also acts as an endocytic receptor in these cells. Earlier studies showed that CD4+ T cells are unable to internalise CD4 due to their expression of protein tyrosine kinase p56lck which stabilises CD4 on the cell surface, while monocytes, which lack this molecule, can internalise CD4 (38). Although endocytosis of HIV-1 virions can occur in DCs (39, 40), it is not known whether CD4 can internalise Env and deposit it in intracellular antigen presentation compartments in these cells. To test their ability to internalise CD4, blood DC subsets were surface-labeled with an anti-CD4 Ab at 4°C followed by a period at 37°C to allow internalisation of the receptor. Residual CD4 Ab remaining on the cell surface was then removed by an acid buffer (pH 3.0), prior to analysis by flow cytometry. At 4°C, CD4 Ab is only bound to the cell surface and the total signal is thus susceptible to acid stripping. At 37°C, the proportion of CD4 Ab that is internalised becomes protected from acid stripping and the signal is retained (Fig. 3a). We found that MDCs, BDCA-3+ DCs and PDCs all internalised CD4 within 60 min, whereas CD4+ T cells maintained their CD4 expression on the surface (Fig. 3b). Furthermore, PDCs internalised Env with similar kinetics to the CD4 Ab, whereas Env remained surface-bound on CD4+ T cells (Fig. 3c). These results demonstrate that DCs which do not utilise CLRs for Env-binding, in particular blood DC subsets including PDCs, are instead able to internalise Env via CD4.
Fig. 3. CD4 mediates Env internalisation in blood DCs, in contrast to T cells.
Enriched MDCs, BDCA-3+ DCs, PDCs and CD4+ T cells were incubated with CD4-PE Ab (SK3 clone) for 20 min at 4°C to allow surface binding, then washed thoroughly. The cells were then incubated at 4°C or 37°C to allow internalisation of the Ab for 0, 60 or 120 min. The cells were then either washed in PBS to preserve the total CD4 signal or acetic acid buffer to strip surface-exposed CD4 Ab. Any remaining signal in the presence of acetic acid indicates the proportion of CD4 that has been internalised. A) Raw FACS data for a representative PDC donor. B) CD4 MFI for donor-matched subsets. C) Donor-matched PDCs or CD4+ T cells were used in the experiment described above but with Env-AF488 substituted as the ligand for CD4. Data are representative of 3 donors.
Uptake of Env via either CLRs or CD4 leads to antigen compartmentalisation in MHC class II+ lysosomes
The cell type and the receptor used for antigen uptake determine the intracellular destination of an antigen and have implications for antigen processing and presentation (5, 8, 9, 41). Given their reported inherent differences in antigen presentation capacity, we investigated if Env was delivered to the same compartment in MDCs and PDCs. MDCs and PDCs were pulsed with Env for up to 90 min and then analysed by confocal microscopy. In both DC subsets, Env was internalised into EEA1+ early endosomes within 10 min but rarely colocalised with Lamp1 (Fig. 4a, top panel). By 90 min the degree of colocalisation with EEA1 had decreased and Env had progressed to colocalise with Lamp1+ lysosomes in both DC subsets (Fig. 4a bottom panel). This staining overlapped with HLA-DR (Fig. 4a bottom panel) and likely represents a compartment for MHC class II loading. From here MHC class II:peptide complexes could be transported to the cell surface and presented to CD4+ T cells. To dissect the intracellular trafficking of Env taken up via CLRs versus CD4, we took advantage of the fact that MR expression could be induced in MDCs by culturing them in GM-CSF. Such cells displayed an equal usage of CLRs and CD4 for Env uptake (Supp. Fig. 1). To study the CLR pathway separately, we pulsed MDCs with the CD4 binding-defective Env-D368R. Conversely, to isolate the CD4 pathway we pre-incubated MDCs with mannan to block CLR-mediated uptake of wt Env. A similar pattern of decreasing colocalisation with EEA1 and increasing colocalisation with Lamp1 and HLA-DR, as described above, was observed for both routes of uptake (Fig. 4b). When Env colocalisation with EEA1 or Lamp1 was quantified, comparing MDCs and PDCs (Fig. 4c) or CLR-dependent versus CD4-dependent Env uptake (Fig. 4d), the same trends were revealed and no statistically significant differences were detected. Although Env rarely appeared to colocalise with Lamp1 visually at early time points, during quantification the diffuse nature of the Lamp1 stain resulted in a higher level of background colocalisation than could be discerned by eye. Importantly, there was increasing colocalisation with Lamp1 over time and for MDCs, this was most pronounced in the comparison between CD4 and CLR-mediated uptake (Fig. 4d). Thus, despite distinctly different DC types or routes of uptake, Env was targeted to MHC class II+ lysosomes.
Fig. 4. Env is delivered to MHC class II+ lysosomes in DCs via both CLRs and CD4.
A) MDCs and PDCs were exposed to 10µg/mL Env-AF488 (green) at 37°C for 10 to 90 min. At various timepoints the cells were washed, fixed and permeabilised, then stained for EEA-1 (red, early endosomes) or Lamp1 (red, lysosomes) and HLA-DR (blue, MHC class II). For visual clarity, only green and red images are merged and colocalisation appears yellow, although the distribution of HLA-DR can be seen to overlap with these areas. Images shown are from 10 min and 90 min. B) MDCs were exposed to 10µg/mL Env-D368R-AF488 as above to follow CLR-mediated uptake or pre-incubated with mannan for 15 min at 37°C followed by exposure to Env-AF488 to follow CD4-mediated uptake. Cells were stained as above. Colocalisation between Env and EEA1 or Lamp1 in C) MDCs versus PDCs and D) CLR-dependent versus CD4-dependent uptake was quantified. Manders’ co-efficients (M1 only) describing the percentage of Env that overlaps with the endolysosomal marker are shown. Between 6–20 cells were analysed for each time point.
CLR-mediated and CD4-mediated endocytosis of Env result in similar kinetics of antigen processing
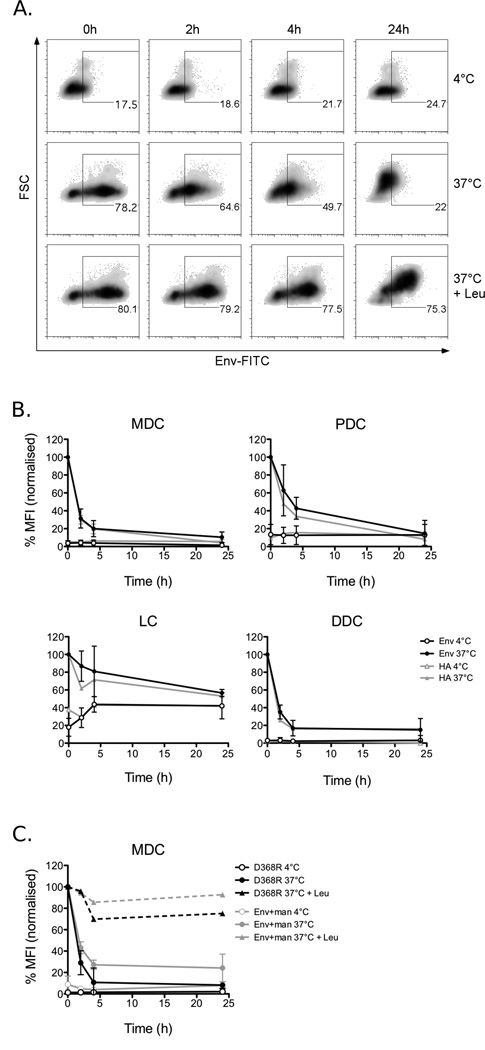
As we found that Env accesses a compartment for potential MHC class II loading of peptides in DCs, we evaluated antigen processing in the different DC subsets. For these studies, we utilised FITC-conjugated Env as this fluorophore is sensitive to the low pH in acidic lysosomal compartments and would therefore be extinguished along with degradation of the antigen. DCs were loaded with antigen (Env or HA for comparison) and the fluorescent signal was tracked over time (Fig. 5a). In both skin and blood DC subsets there was a rapid decay of the Env-FITC signal within 2–4 h followed by relative preservation of a fraction of the antigen (approximately 10%) for 24 h (Fig. 5b). This rate of degradation was previously described for other antigens in primary DCs (42–44) and is similar to the degradation kinetics observed for HIV-1 virions (45). The rate of Env degradation also closely mirrored that of HA (Fig. 5b). Furthermore, when the CLR and CD4-mediated pathways were studied separately in MDCs by using the conditions described above, there were no significant differences in the rate of degradation of Env internalised via either route (Fig. 5c). To confirm that this decay represented real proteolytic degradation of Env and not simply quenching of the FITC signal, the experiment was performed in MDCs in the presence of leupeptin, a competitive lysosomal protease inhibitor, or at 4°C where antigen is not internalised. Under these conditions, the majority of the Env-FITC signal was preserved (Fig. 5a, c) indicating that the observed decay is due to proteolytic digestion of the protein. Also, Western blot analysis for the presence of Env in cell lysates, collected at the indicated time points, confirmed that most of the protein was degraded within 2–4 hrs and that this was inhibited by leupeptin (data not shown). Thus, Env internalised via CD4 or CLRs and by all DC subsets analysed here is efficiently degraded in MHC class II+ compartments, suggesting the potential for antigen presentation.
Fig. 5. Both CLR-mediated and CD4-mediated endocytosis of Env result in similar kinetics of antigen processing.
Cells were incubated with 10µg/mL FITC-labeled Env or HA for 90 min at 4°C or 37°C, then washed twice and cultured at 4°C or 37°C for up to 24h. In some cases, MDCs were pre-incubated and the experiment carried out in the presence of 1mM leupeptin (Leu) to inhibit proteolytic degradation. Samples were then stained with DC markers for analysis by flow cytometry and gated on HLA-DR+/CD11c+ (MDCs), CD123+ (PDCs), CD1a+ (LCs) or HLA-DR+ (DDCs). A) Representative MDC donor showing the percentage of positive cells. B) The MFI for each timepoint was normalised to the 0 min 37°C control for each Ag and the data shown here are the mean ± SD for for 3–5 donors for Env and 1 donor for HA. C) Alternatively, MDCs were incubated with Env in the presence of mannan or incubated with Env-D368R to isolate degradation via CD4 and CLR-mediated uptake pathways respectively. Leupeptin was also included in these experiments as a control. Data here are the mean ± SD of 2 donors.
CD4-mediated endocytosis of Env results in MHC class II presentation to CD4+ T cells
CLR-mediated antigen uptake is known to result in antigen presentation on MHC class II by DCs (46–49), but it is not known whether CD4-mediated uptake has the same result. We investigated this in the context of Env in the blood DC subsets. To enable these studies, we designed a fusion protein between Env and the immunodominant cytomegalovirus (CMV) protein pp65 (65 kDa lower matrix phosphoprotein) as an epitope tag. The latter is a tegument protein, not exposed on the surface of CMV and is not involved in virus binding to cells. We reasoned that if the Env-pp65 fusion protein was targeted to, and endocytosed via, the Env receptors, namely CD4 in blood DCs, it would follow a similar processing route to Env alone. As detectable pre-existing memory T cell responses to pp65 are common in the human population, CD4+ T cell memory responses to the pp65 epitope tag could then be measured as a surrogate read-out for Env presentation. The wt (Env-pp65) and the CD4 binding-defective (Env-D368R-pp65) fusion proteins were characterised biochemically prior to the antigen presentation assays (Supp. Fig. 3). The fusion proteins were 180–190 kDa, glycosylated and preserved both pp65 epitopes and key Env epitopes. Critically, only wt Env-pp65 was recognised by the CD4 binding site-directed monoclonal Ab b12. When binding of fluorescently-conjugated versions of the fusion proteins was assessed in MDCs and PDCs the results were similar to those observed for Env and Env-D368R (Fig. 6a). Both MDCs and PDCs were able to bind wt Env-pp65 at 4°C, with PDCs demonstrating slightly higher binding, as was observed for Env alone. Binding of the Env-D368R-pp65 fusion protein was markedly reduced although still detectable in MDCs whereas in PDCs binding was abolished, suggesting that CD4 mediates the majority of the uptake of the fusion proteins in these cell types. Recombinant pp65 protein alone was also bound by a small proportion of MDCs, but there was no detectable binding in PDCs. Neither the fusion proteins, nor pp65 induced maturation of MDCs or PDCs in terms of upregulation of co-stimulatory molecules, consistent with previous studies of Env (50, 51), while in contrast, a positive control TLR7/8-ligand effectively stimulated DC maturation (Fig. 6b).
Fig. 6. CD4-mediated endocytosis of Env in blood DCs, including PDCs, results in MHC class II presentation to CD4+ T cells.
A) MDCs and PDCs were pulsed with the Env-pp65 or Env-D368R-pp65 fusion proteins, or recombinant pp65, each labeled with AF488, for 1h at 4°C. The degree of binding was measured by flow cytometry. B) To check for induction of maturation, MDCs and PDCs were exposed to the fusion proteins or pp65 at 4°C for 1h then washed and cultured overnight. Maturation was measured by staining for co-stimulatory molecules. Cells cultured overnight with TLR7/8-ligand acted as a positive control. C) Highly purified DC subsets were obtained by flow cytometric sorting and their immature status was confirmed by staining for co-stimulatory molecules and HLA-DR. D) Comparison of the expression levels of HLA-DR between the DC subsets in three matched donors. E) Sorted, immature MDCs, BDCA-3+ DCs and PDCs were pulsed with overlapping 15 mer peptides from CMV pp65 (10–fold serial dilutions) or F) Env-pp65 or Env-D368R-pp65 at 4°C for 1h. As controls, the cells were pulsed with no Ag or recombinant pp65. Excess Ag was washed away and the DCs then cultured with autologous CD4+ T cells at a ratio 1:10 in the presence of brefeldin A. After 16 h the activation of CD4+ T cells was measured by the production of intracellular cytokines (IFN-γ, TNF and IL-2), assessed by flow cytometry. The percentage shown is of live CD11c- or CD123-/HLA-DR-/CD4+ cells. MDCs exposed to antigen then fixed with 1% paraformaldehyde prior to culture with CD4+ T cells were also used to control for the absence of pp65 peptides in the fusion protein preparations. G) The mean fold change in the percentage of cytokine-positive cells when stimulated with Env-D368R-pp65 compared to Env-pp65 is shown (mean+SD, n=3). **p<0.01, ***p<0.001.
To specifically study whether CD4-mediated uptake can result in antigen presentation, we sorted the blood DC subsets to high purity by flow cytometry. Following this isolation procedure, all subsets displayed an immature phenotype with low expression of the co-stimulatory molecules CD40 and CD86 (Fig. 6c), which again could be induced by stimulation with a TLR7/8-ligand (Fig. 6b). HLA-DR expression was highest on MDCs and BDCA-3+ DCs, as expected, and approximately half a log lower on PDCs (Fig. 6d), suggesting the PDCs have an inherent disadvantage when it comes to efficient MHC class II antigen presentation. Reflecting this, we observed that BDCA-3+ DCs and MDCs stimulated CD4+ T cells more efficiently than PDCs via MHC class II when surface pulsed with overlapping pp65 peptides (Fig. 6e). To test whether CD4-mediated uptake of Env leads to antigen presentation, we pulsed the respective DC subsets with the fusion proteins at 4°C to avoid non-receptor mediated uptake mechanisms, then washed and co-cultured them with autologous CD4+ T cells. Pp65-specific T cells responding to antigen presentation by the DCs were detected by intracellular cytokine staining for IFN-γ, TNF and IL-2. MDCs that were exposed to the fusion proteins, then fixed prior to culture with CD4+ T cells did not induce any T cell activation, demonstrating the absence of contaminating MHC class II-restricted pp65 peptides in the protein preparations. Live MDCs were capable of presenting both Env-pp65 and Env-D368R-pp65 but presentation of the latter reduced the numbers of responding T cells by half, suggesting that CD4 mediates some but not all of the uptake leading to antigen presentation (Fig. 6f–g). Both BDCA-3+ DCs and PDCs efficiently presented Env-pp65 but in contrast to MDCs, their presentation of Env-D368R-pp65 was extremely poor, demonstrating that CD4-mediated Env uptake in these cells is the main pathway leading to processing and presentation to CD4+ T cells (Fig. 6f–g). Furthermore, despite differences in MHC class II expression (Fig. 6d) and reported intrinsic differences in MHC class II antigen presentation capacity, donor-matched MDC, BDCA-3+ DC and PDC subsets pulsed with Env-pp65 stimulated similar percentages of CD4+ T cells. Thus, these data demonstrate internalisation of Env via CD4 in DCs represents a functional pathway that results in antigen processing and presentation to CD4+ T cells.
Discussion
Due to their anatomical distribution in peripheral tissues, DCs are likely to be one of the first immune cells to come into contact with vaccine antigens after delivery. Through their unique capacity to transport antigen to the lymph nodes and prime naïve CD4+ T cells they provide the critical link between the administration of a vaccine and the development of a specific response. Thus, the efficiency with which DCs take up the vaccine antigen and present peptides to CD4+ T cells is considered central to the induction of potent vaccine responses. In this study, we analysed the interactions between multiple primary human DC subsets and soluble HIV-1 Env as a HIV-1 immunogen. We show that skin and blood DC subsets take up Env to varying degrees via different CLRs and CD4 respectively, dictated by their respective receptor expression repertoire, supporting previous work by Turville et al (36). Strikingly, PDCs, which are generally poor at uptake of soluble antigen and are thought to only present antigen to CD4+ T cells under certain conditions, showed a particularly high capacity for internalisation of Env via CD4. We further demonstrated that this route is a functional antigen uptake pathway in multiple DC subsets to deliver Env to the endolysosomal system where it is degraded and subsequently presented to CD4+ T cells.
Whereas CLRs are known pattern recognition receptors utilised by DCs for the capture of a broad range of antigens, facilitating their role in immune surveillance, CD4 does not have an established role in antigen capture and processing. Specific CLRs such as the mannose receptor, DC-SIGN and Langerin have a higher functional affinity for Env than CD4 does, which may include avidity effects, (27) and this has been suggested as a mechanism by which DCs expressing CLRs can redirect the virus from conventional infection towards endocytosis and degradation (52). Alternatively, CLR-mediated uptake can lead to trans infection where internalised virions are neither degraded nor establish a productive infection but are transferred to other susceptible cells (28, 53). While DCs are unlikely to be important in clearance of the virus, their capacity to endocytose, degrade and subsequently present HIV-1 antigens to T cells is critical for the development of HIV-specific adaptive immune responses. DC subsets with low expression of high-mannose binding CLRs such as blood DCs may therefore be more vulnerable to infection and/or utilise CD4 more readily as a receptor to endocytose the virus. The latter is supported by the finding that blocking BDCA-2, a CLR expressed on PDCs with binding affinity for Env, was shown not to reduce endocytosis of HIV-1 virions (40).
Extensive efforts have been directed towards targeting vaccine antigens to DCs via specific CLRs to improve antigen uptake and presentation (54–57). This strategy can result in enhancement of T and B cell responses by orders of magnitude and reduce the dose of antigen required in a vaccine setting. However, depending on the choice of targeting receptor and the expression pattern of that receptor on DC subsets, the immunological outcome will likely differ. MDCs are generally superior to PDCs for MHC class II antigen presentation (58–60), while BDCA-3+ DCs excel at cross-presentation on MHC class I molecules (3, 61). Restricted receptor expression and lower endocytic activity are thought to account for the less efficient uptake and presentation of exogenous antigen by PDCs than MDCs (62). In the case of Env, its high glycosylation naturally targets it to DCs via their CLRs. In addition, the data presented here suggest that the high-affinity interaction between Env and CD4, which is highly expressed on PDCs, also enables efficient uptake of Env in these cells.
Apart from antigen uptake, the weaker capacity of PDCs for exogenous antigen presentation on MHC class II has been related to their failure to reduce the turnover rate of MHC class II molecules, in contrast to other DC subsets. As a consequence, PDCs are unable to generate stable MHC class II:peptide complexes that facilitate extended engagement of CD4+ T cells and optimal T cell activation (63, 64). This is consistent with what we observed in peptide dose response experiments. However, under the right conditions, such as when provided with temporal toll-like receptor stimulation (5), antigen complexed to immune serum (17) or specific receptor usage (18, 65) PDCs have been shown to function well as antigen presenting cells and even comparably to MDCs. In the current study, we found that donor-matched PDCs, MDCs and BDCA-3+ DCs presented Env-pp65 on MHC class II and stimulated CD4+ T cells with similar efficiencies when identical experimental conditions were used. The enhanced presentation capacity of PDCs in this instance compared to earlier studies is likely a result of their efficient Env uptake, which was unusually high compared to other protein antigens. This suggests that CD4-mediated uptake of Env by DCs facilitates presentation and could contribute to the generation of Env- specific CD4+ T cell responses by blood DCs in particular.
These results highlight the possibility that PDCs, which show limited CLR expression but relatively high CD4 expression, may play a special role as antigen-presenting cells for Env compared to other antigens. We, and others, have previously shown that PDCs play a unique role in supporting B cell responses in vitro (13–16). In the presence of TLR stimulation or BCR ligation and T cell help, PDC-derived IFNα supports the differentiation of B cells into Ab secreting cells and enhances their proliferation (13, 14). The results presented here suggest that PDCs can also efficiently stimulate CD4+ T cells. Thus, the influence of PDCs on responses elicited by an Env subunit vaccine could be multifactorial. However, an important consideration is that in vivo there are likely to be multiple Env uptake pathways in operation. In fact, we recently reported that immunisation of non-human primates with CD4 binding-competent or CD4-binding defective Env trimers resulted in Env-specific T cell responses, as well as B cell responses, of comparable magnitude (23). This suggests that ablating CD4-mediated uptake and therefore potentially the ability of PDCs to contribute to Env presentation in vivo does not compromise peak CD4+ T cell helper response. However, more subtle effects of the Env-CD4 interaction on vaccine-induced immune responses in the absence of a strong adjuvant were not investigated in that study. Furthermore, others have shown that Env binding to CLRs on DCs stimulates an immunosuppressive response (66) and DC apoptosis (67), and blocking or removing the mannose moieties on Env, and thus precluding endocytosis via CLRs, enhanced Env-specific humoral responses in vivo (68, 69). Thus, uptake and presentation pathways not relying on CLRs may be relevant to Env vaccination.
In summary, we have identified a pathway by which DCs expressing limited or no Env-binding CLRs may be involved in binding and uptake of Env-based vaccines and the subsequent priming of a naïve CD4+ T cell response. This CD4-mediated pathway may be especially relevant in the context of intramuscular vaccinations where blood DCs are likely to constitute the main infiltrating DC population at the site of inoculation.
Supplementary Material
Acknowledgements
The authors thank Drs. Barney Graham and Mario Roederer, and Ms. Elizabeth Thompson (Vaccine Research Center, NIH, USA) and Dr. Emily Bond (Karolinska Institutet) for technical help and valuable advice.
This study was supported by grants from the Swedish International Development Agency (Sida) to AS-S, GKH and KL as well as the Swedish Research Council and the Swedish Governmental Agency for Innovation Systems (Vinnova) to KL. KJS and FL are recipients of scholarships from the Swedish Society of Medicine, the Swedish Society of Medical Research and the Fernström Foundation. MNF is supported by an amfAR Mathilde Krim Fellowship in Basic Biomedical Research.
Non-standard abbreviations
- PDC
plasmacytoid dendritic cell
- MDC
myeloid dendritic cell
- LC
Langerhans cell
- DDC
dermal dendritic cell
- Env
envelope glycoprotein
Footnotes
Conflict of Interest
The authors state no conflict of interest.
References
- 1.Karlsson Hedestam G, Fouchier R, Phogat S, Burton D, Sodroski J, Wyatt R. The challenges of eliciting neutralizing antibodies to HIV-1 and to influenza virus. Nature reviews. Microbiology. 2008;6:143–198. doi: 10.1038/nrmicro1819. [DOI] [PubMed] [Google Scholar]
- 2.Kwong P, Wilson I. HIV-1 and influenza antibodies: seeing antigens in new ways. Nature immunology. 2009;10:573–581. doi: 10.1038/ni.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jongbloed S, Kassianos A, McDonald K, Clark G, Ju X, Angel C, Chen C-J, Dunbar P, Wadley R, Jeet V, Vulink A, Hart D, Radford K. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. The Journal of experimental medicine. 2010;207:1247–1307. doi: 10.1084/jem.20092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bond E, Liang F, Sandgren K, Smed-Sorensen A, Bergman P, Brighenti S, Adams W, Betemariam S, Rangaka M, Lange C, Wilkinson R, Andersson J, Loré K. Plasmacytoid dendritic cells infiltrate the skin in positive tuberculin skin test indurations. The Journal of investigative dermatology. 2012;132:114–137. doi: 10.1038/jid.2011.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kool M, Geurtsvankessel C, Muskens F, Madeira FB, van Nimwegen M, Kuipers H, Thielemans K, Hoogsteden HC, Hammad H, Lambrecht BN. Facilitated antigen uptake and timed exposure to TLR ligands dictate the antigen-presenting potential of plasmacytoid DCs. Journal of leukocyte biology. 2011;90:1177–1190. doi: 10.1189/jlb.0610342. [DOI] [PubMed] [Google Scholar]
- 6.Robinson S, Patterson S, English N, Davies D, Knight S, Reid C. Human peripheral blood contains two distinct lineages of dendritic cells. European journal of immunology. 1999;29:2769–2847. doi: 10.1002/(SICI)1521-4141(199909)29:09<2769::AID-IMMU2769>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 7.Tel J, Lambeck AJ, Cruz LJ, Tacken PJ, de Vries IJ, Figdor CG. Human plasmacytoid dendritic cells phagocytose, process, and present exogenous particulate antigen. Journal of immunology. 2010;184:4276–4283. doi: 10.4049/jimmunol.0903286. [DOI] [PubMed] [Google Scholar]
- 8.Burgdorf S, Kautz A, Bohnert V, Knolle P, Kurts C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science (New York, N.Y.) 2007;316:612–618. doi: 10.1126/science.1137971. [DOI] [PubMed] [Google Scholar]
- 9.Delamarre Ll, Mellman I. Harnessing dendritic cells for immunotherapy. Seminars in immunology. 2011;23:2–13. doi: 10.1016/j.smim.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Chatterjee B, Smed-Sorensen A, Cohn L, Chalouni C, Vandlen R, Lee BC, Widger J, Keler T, Delamarre L, Mellman I. Internalization and endosomal degradation of receptor-bound antigens regulate the efficiency of cross presentation by human dendritic cells. Blood. 2012 doi: 10.1182/blood-2012-01-402370. [DOI] [PubMed] [Google Scholar]
- 11.Dudziak D, Kamphorst A, Heidkamp G, Buchholz V, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee H-W, Park C, Steinman R, Nussenzweig M. Differential antigen processing by dendritic cell subsets in vivo. Science (New York, N.Y.) 2007;315:107–118. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 12.Robbins S, Walzer T, Dembele D, Thibault C, Defays A, Bessou G, Xu H, Vivier E, Sellars M, Pierre P, Sharp F, Chan S, Kastner P, Dalod M. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome biology. 2008;9 doi: 10.1186/gb-2008-9-1-r17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douagi I, Gujer C, Sundling C, Adams WC, Smed-Sorensen A, Seder RA, Karlsson Hedestam GB, Lore K. Human B cell responses to TLR ligands are differentially modulated by myeloid and plasmacytoid dendritic cells. Journal of immunology. 2009;182:1991–2001. doi: 10.4049/jimmunol.0802257. [DOI] [PubMed] [Google Scholar]
- 14.Gujer C, Sandgren K, Douagi I, Adams W, Sundling C, Smed-Sorensen A, Seder R, Karlsson Hedestam G, Loré K. IFN-α produced by human plasmacytoid dendritic cells enhances T cell-dependent naïve B cell differentiation. Journal of leukocyte biology. 2011;89:811–832. doi: 10.1189/jlb.0810460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 16.Shaw J, Wang YH, Ito T, Arima K, Liu YJ. Plasmacytoid dendritic cells regulate B-cell growth and differentiation via CD70. Blood. 2010;115:3051–3057. doi: 10.1182/blood-2009-08-239145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benitez-Ribas D, Adema GJ, Winkels G, Klasen IS, Punt CJ, Figdor CG, de Vries IJ. Plasmacytoid dendritic cells of melanoma patients present exogenous proteins to CD4+ T cells after Fc gamma RII-mediated uptake. The Journal of experimental medicine. 2006;203:1629–1635. doi: 10.1084/jem.20052364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer-Wentrup F, Benitez-Ribas D, Tacken PJ, Punt CJ, Figdor CG, de Vries IJ, Adema GJ. Targeting DCIR on human plasmacytoid dendritic cells results in antigen presentation and inhibits IFN-alpha production. Blood. 2008;111:4245–4253. doi: 10.1182/blood-2007-03-081398. [DOI] [PubMed] [Google Scholar]
- 19.Lore K, Smed-Sorensen A, Vasudevan J, Mascola JR, Koup RA. Myeloid and plasmacytoid dendritic cells transfer HIV-1 preferentially to antigen-specific CD4+ T cells. J Exp Med. 2005;201:2023–2033. doi: 10.1084/jem.20042413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adams WC, Bond E, Havenga MJ, Holterman L, Goudsmit J, Karlsson Hedestam GB, Koup RA, Lore K. Adenovirus serotype 5 infects human dendritic cells via a coxsackievirus-adenovirus receptor-independent receptor pathway mediated by lactoferrin and DC-SIGN. The Journal of general virology. 2009;90:1600–1610. doi: 10.1099/vir.0.008342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bond E, Adams WC, Smed-Sorensen A, Sandgren KJ, Perbeck L, Hofmann A, Andersson J, Lore K. Techniques for time-efficient isolation of human skin dendritic cell subsets and assessment of their antigen uptake capacity. J Immunol Methods. 2009 doi: 10.1016/j.jim.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Olshevsky U, Helseth E, Furman C, Li J, Haseltine W, Sodroski J. Identification of individual human immunodeficiency virus type 1 gp120 amino acids important for CD4 receptor binding. Journal of virology. 1990;64:5701–5707. doi: 10.1128/jvi.64.12.5701-5707.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Douagi I, Forsell MN, Sundling C, O'Dell S, Feng Y, Dosenovic P, Li Y, Seder R, Lore K, Mascola JR, Wyatt RT, Karlsson Hedestam GB. Influence of novel CD4 binding-defective HIV-1 envelope glycoprotein immunogens on neutralizing antibody and T-cell responses in nonhuman primates. Journal of virology. 2010;84:1683–1695. doi: 10.1128/JVI.01896-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forsell MN, Dey B, Morner A, Svehla K, O'Dell S, Hogerkorp CM, Voss G, Thorstensson R, Shaw GM, Mascola JR, Karlsson Hedestam GB, Wyatt RT. B cell recognition of the conserved HIV-1 co-receptor binding site is altered by endogenous primate CD4. PLoS pathogens. 2008;4:e1000171. doi: 10.1371/journal.ppat.1000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sundling C, O'Dell S, Douagi I, Forsell MN, Morner A, Lore K, Mascola JR, Wyatt RT, Karlsson Hedestam GB. Immunization with wild-type or CD4-binding-defective HIV-1 Env trimers reduces viremia equivalently following heterologous challenge with simian-human immunodeficiency virus. Journal of virology. 2010;84:9086–9095. doi: 10.1128/JVI.01015-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolte S, Cordelieres FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224:213–232. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- 27.Turville SG, Arthos J, Donald KM, Lynch G, Naif H, Clark G, Hart D, Cunningham AL. HIV gp120 receptors on human dendritic cells. Blood. 2001;98:2482–2488. doi: 10.1182/blood.v98.8.2482. [DOI] [PubMed] [Google Scholar]
- 28.Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet HS, KewalRamani VN, Littman DR, Figdor CG, van Kooyk Y. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 29.Hatsukari I, Singh P, Hitosugi N, Messmer D, Valderrama E, Teichberg S, Chaung W, Gross E, Schmidtmayerova H, Singhal PC. DEC-205-mediated internalization of HIV-1 results in the establishment of silent infection in renal tubular cells. J Am Soc Nephrol. 2007;18:780–787. doi: 10.1681/ASN.2006121307. [DOI] [PubMed] [Google Scholar]
- 30.Lambert AA, Gilbert C, Richard M, Beaulieu AD, Tremblay MJ. The C-type lectin surface receptor DCIR acts as a new attachment factor for HIV-1 in dendritic cells and contributes to trans- and cis-infection pathways. Blood. 2008;112:1299–1307. doi: 10.1182/blood-2008-01-136473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Panfilis G, Manara GC, Ferrari C, Torresani C. Simultaneous colloidal gold immunoelectronmicroscopy labeling of CD1a, HLA-DR, and CD4 surface antigens of human epidermal Langerhans cells. The Journal of investigative dermatology. 1988;91:547–552. doi: 10.1111/1523-1747.ep12476912. [DOI] [PubMed] [Google Scholar]
- 32.Lynch GW, Slaytor EK, Elliott FD, Saurajen A, Turville SG, Sloane AJ, Cameron PU, Cunningham AL, Halliday GM. CD4 is expressed by epidermal Langerhans' cells predominantly as covalent dimers. Exp Dermatol. 2003;12:700–711. doi: 10.1034/j.1600-0625.2003.00078.x. [DOI] [PubMed] [Google Scholar]
- 33.Wood GG, Warner NL, Warnke RA. Anti-Leu-3/T4 antibodies react with cells of monocyte/macrophage and Langerhans lineage. Journal of immunology. 1983;131:212–216. [PubMed] [Google Scholar]
- 34.Martinelli E, Cicala C, Van Ryk D, Goode DJ, Macleod K, Arthos J, Fauci AS. HIV-1 gp120 inhibits TLR9-mediated activation and IFN-{alpha} secretion in plasmacytoid dendritic cells. Proc Natl Acad Sci U S A. 2007;104:3396–3401. doi: 10.1073/pnas.0611353104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harman AN, Bye CR, Nasr N, Sandgren KJ, Kim M, Mercier SK, Botting RA, Lewin SR, Cunningham AL, Cameron PU. Identification of lineage relationships and novel markers of blood and skin human dendritic cells. J Immunol. 2013;190:66–79. doi: 10.4049/jimmunol.1200779. [DOI] [PubMed] [Google Scholar]
- 36.Turville S, Cameron P, Handley A, Lin G, Pohlmann S, Doms R, Cunningham A. Diversity of receptors binding HIV on dendritic cell subsets. Nature immunology. 2002;3:975–1058. doi: 10.1038/ni841. [DOI] [PubMed] [Google Scholar]
- 37.Yang X, Lee J, Mahony EM, Kwong PD, Wyatt R, Sodroski J. Highly stable trimers formed by human immunodeficiency virus type 1 envelope glycoproteins fused with the trimeric motif of T4 bacteriophage fibritin. Journal of virology. 2002;76:4634–4642. doi: 10.1128/JVI.76.9.4634-4642.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pelchen-Matthews A, da Silva RP, Bijlmakers MJ, Signoret N, Gordon S, Marsh M. Lack of p56lck expression correlates with CD4 endocytosis in primary lymphoid and myeloid cells. European journal of immunology. 1998;28:3639–3647. doi: 10.1002/(SICI)1521-4141(199811)28:11<3639::AID-IMMU3639>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 39.Beignon AS, McKenna K, Skoberne M, Manches O, DaSilva I, Kavanagh DG, Larsson M, Gorelick RJ, Lifson JD, Bhardwaj N. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest. 2005;115:3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pritschet K, Donhauser N, Schuster P, Ries M, Haupt S, Kittan NA, Korn K, Pohlmann S, Holland G, Bannert N, Bogner E, Schmidt B. CD4- and dynamin-dependent endocytosis of HIV-1 into plasmacytoid dendritic cells. Virology. 2012;423:152–164. doi: 10.1016/j.virol.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 41.Kamphorst A, Guermonprez P, Dudziak D, Nussenzweig M. Route of antigen uptake differentially impacts presentation by dendritic cells and activated monocytes. Journal of immunology. 2010;185:3426–3461. doi: 10.4049/jimmunol.1001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delamarre Ll, Pack M, Chang H, Mellman I, Trombetta E. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science (New York, N.Y.) 2005;307:1630–1634. doi: 10.1126/science.1108003. [DOI] [PubMed] [Google Scholar]
- 43.Trombetta E, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annual review of immunology. 2005;23:975–2003. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 44.Savina A, Amigorena S. Phagocytosis and antigen presentation in dendritic cells. Immunol Rev. 2007;219:143–156. doi: 10.1111/j.1600-065X.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- 45.Turville S, Santos J, Frank I, Cameron P, Wilkinson J, Miranda-Saksena M, Dable J, Stossel H, Romani N, Piatak M, Lifson J, Pope M, Cunningham A. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood. 2004;103:2170–2179. doi: 10.1182/blood-2003-09-3129. [DOI] [PubMed] [Google Scholar]
- 46.Engering A, Geijtenbeek TB, van Vliet SJ, Wijers M, van Liempt E, Demaurex N, Lanzavecchia A, Fransen J, Figdor CG, Piguet V, van Kooyk Y. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. Journal of immunology. 2002;168:2118–2126. doi: 10.4049/jimmunol.168.5.2118. [DOI] [PubMed] [Google Scholar]
- 47.Engering AJ, Cella M, Fluitsma DM, Hoefsmit EC, Lanzavecchia A, Pieters J. Mannose receptor mediated antigen uptake and presentation in human dendritic cells. Adv Exp Med Biol. 1997;417:183–187. doi: 10.1007/978-1-4757-9966-8_31. [DOI] [PubMed] [Google Scholar]
- 48.Idoyaga J, Cheong C, Suda K, Suda N, Kim JY, Lee H, Park CG, Steinman RM. Cutting edge: langerin/CD207 receptor on dendritic cells mediates efficient antigen presentation on MHC I and II products in vivo. Journal of immunology. 2008;180:3647–3650. doi: 10.4049/jimmunol.180.6.3647. [DOI] [PubMed] [Google Scholar]
- 49.Tan MC, Mommaas AM, Drijfhout JW, Jordens R, Onderwater JJ, Verwoerd D, Mulder AA, van der Heiden AN, Ottenhoff TH, Cella M, Tulp A, Neefjes JJ, Koning F. Mannose receptor mediated uptake of antigens strongly enhances HLA-class II restricted antigen presentation by cultured dendritic cells. Adv Exp Med Biol. 1997;417:171–174. doi: 10.1007/978-1-4757-9966-8_28. [DOI] [PubMed] [Google Scholar]
- 50.Harman AN, Kraus M, Bye CR, Byth K, Turville SG, Tang O, Mercier SK, Nasr N, Stern JL, Slobedman B, Driessen C, Cunningham AL. HIV-1-infected dendritic cells show 2 phases of gene expression changes, with lysosomal enzyme activity decreased during the second phase. Blood. 2009;114:85–94. doi: 10.1182/blood-2008-12-194845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harman AN, Wilkinson J, Bye CR, Bosnjak L, Stern JL, Nicholle M, Lai J, Cunningham AL. HIV induces maturation of monocyte-derived dendritic cells and Langerhans cells. J Immunol. 2006;177:7103–7113. doi: 10.4049/jimmunol.177.10.7103. [DOI] [PubMed] [Google Scholar]
- 52.de Witte L, Nabatov A, Pion M, Fluitsma D, de Jong M, de Gruijl T, Piguet V, van Kooyk Y, Geijtenbeek T. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nature medicine. 2007;13:367–438. doi: 10.1038/nm1541. [DOI] [PubMed] [Google Scholar]
- 53.Turville S, Wilkinson J, Cameron P, Dable J, Cunningham AL. The role of dendritic cell C-type lectin receptors in HIV pathogenesis. Journal of leukocyte biology. 2003;74:710–718. doi: 10.1189/jlb.0503208. [DOI] [PubMed] [Google Scholar]
- 54.Caminschi I, Shortman K. Boosting antibody responses by targeting antigens to dendritic cells. Trends Immunol. 2012;33:71–77. doi: 10.1016/j.it.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 55.Tacken PJ, Figdor CG. Targeted antigen delivery and activation of dendritic cells in vivo: steps towards cost effective vaccines. Seminars in immunology. 2011;23:12–20. doi: 10.1016/j.smim.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 56.Lahoud MH, Ahmet F, Kitsoulis S, Wan SS, Vremec D, Lee CN, Phipson B, Shi W, Smyth GK, Lew AM, Kato Y, Mueller SN, Davey GM, Heath WR, Shortman K, Caminschi I. Targeting antigen to mouse dendritic cells via Clec9A induces potent CD4 T cell responses biased toward a follicular helper phenotype. Journal of immunology. 2011;187:842–850. doi: 10.4049/jimmunol.1101176. [DOI] [PubMed] [Google Scholar]
- 57.Boscardin SB, Hafalla JC, Masilamani RF, Kamphorst AO, Zebroski HA, Rai U, Morrot A, Zavala F, Steinman RM, Nussenzweig RS, Nussenzweig MC. Antigen targeting to dendritic cells elicits long-lived T cell help for antibody responses. The Journal of experimental medicine. 2006;203:599–606. doi: 10.1084/jem.20051639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lore K, Adams WC, Havenga MJ, Precopio ML, Holterman L, Goudsmit J, Koup RA. Myeloid and plasmacytoid dendritic cells are susceptible to recombinant adenovirus vectors and stimulate polyfunctional memory T cell responses. J Immunol. 2007;179:1721–1729. doi: 10.4049/jimmunol.179.3.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lore K, Betts MR, Brenchley JM, Kuruppu J, Khojasteh S, Perfetto S, Roederer M, Seder RA, Koup RA. Toll-like receptor ligands modulate dendritic cells to augment cytomegalovirus- and HIV-1-specific T cell responses. J Immunol. 2003;171:4320–4328. doi: 10.4049/jimmunol.171.8.4320. [DOI] [PubMed] [Google Scholar]
- 60.Di Pucchio T, Chatterjee B, Smed-Sorensen A, Clayton S, Palazzo A, Montes M, Xue Y, Mellman I, Banchereau J, Connolly JE. Direct proteasome-independent cross-presentation of viral antigen by plasmacytoid dendritic cells on major histocompatibility complex class I. Nature immunology. 2008;9:551–557. doi: 10.1038/ni.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bachem A, Guttler S, Hartung E, Ebstein F, Schaefer M, Tannert A, Salama A, Movassaghi K, Opitz C, Mages HW, Henn V, Kloetzel PM, Gurka S, Kroczek RA. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. The Journal of experimental medicine. 2010;207:1273–1281. doi: 10.1084/jem.20100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Villadangos JA, Young L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity. 2008;29:352–361. doi: 10.1016/j.immuni.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 63.Sadaka C, Marloie-Provost MA, Soumelis V, Benaroch P. Developmental regulation of MHC II expression and transport in human plasmacytoid-derived dendritic cells. Blood. 2009;113:2127–2135. doi: 10.1182/blood-2008-10-178152. [DOI] [PubMed] [Google Scholar]
- 64.Young LJ, Wilson NS, Schnorrer P, Proietto A, ten Broeke T, Matsuki Y, Mount AM, Belz GT, O'Keeffe M, Ohmura-Hoshino M, Ishido S, Stoorvogel W, Heath WR, Shortman K, Villadangos JA. Differential MHC class II synthesis and ubiquitination confers distinct antigen-presenting properties on conventional and plasmacytoid dendritic cells. Nature immunology. 2008;9:1244–1252. doi: 10.1038/ni.1665. [DOI] [PubMed] [Google Scholar]
- 65.Tel J, Benitez-Ribas D, Hoosemans S, Cambi A, Adema GJ, Figdor CG, Tacken PJ, de Vries IJ. DEC-205 mediates antigen uptake and presentation by both resting and activated human plasmacytoid dendritic cells. European journal of immunology. 2011;41:1014–1023. doi: 10.1002/eji.201040790. [DOI] [PubMed] [Google Scholar]
- 66.Shan M, Klasse PJ, Banerjee K, Dey AK, Iyer SP, Dionisio R, Charles D, Campbell-Gardener L, Olson WC, Sanders RW, Moore JP. HIV-1 gp120 mannoses induce immunosuppressive responses from dendritic cells. PLoS Pathog. 2007;3:e169. doi: 10.1371/journal.ppat.0030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen Y, Hwang SL, Chan VS, Chung NP, Wang SR, Li Z, Ma J, Lin CW, Hsieh YJ, Chang KP, Kung SS, Wu YC, Chu CW, Tai HT, Gao GF, Zheng B, Yokoyama KK, Austyn JM, Lin CL. Binding of HIV-1 gp120 to DC-SIGN Promotes ASK-1-Dependent Activation-Induced Apoptosis of Human Dendritic Cells. PLoS pathogens. 2013;9:e1003100. doi: 10.1371/journal.ppat.1003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Banerjee K, Andjelic S, Klasse PJ, Kang Y, Sanders RW, Michael E, Durso RJ, Ketas TJ, Olson WC, Moore JP. Enzymatic removal of mannose moieties can increase the immune response to HIV-1 gp120 in vivo. Virology. 2009;389:108–121. doi: 10.1016/j.virol.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Banerjee K, Michael E, Eggink D, van Montfort T, Lasnik AB, Palmer KE, Sanders RW, Moore JP, Klasse PJ. Occluding the mannose moieties on human immunodeficiency virus type 1 gp120 with griffithsin improves the antibody responses to both proteins in mice. AIDS Res Hum Retroviruses. 2012;28:206–214. doi: 10.1089/aid.2011.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.