Table 2.
Scope of the enantioselective carbocyclization–borylation reaction.[a]
| Entry | Enallene | Carbocycle | Time [h] | Yield [%][b] | ee [%][c] |
|---|---|---|---|---|---|
| 1 | 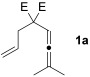 |
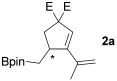 |
42 | 93 | 84 |
| 2 | 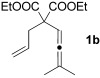 |
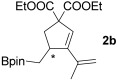 |
42 | 88 | 84 |
| 3 | 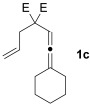 |
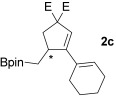 |
84 | 79 | 93 |
| 4 | 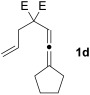 |
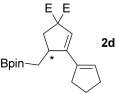 |
72 | 83 | 92 |
| 5 | 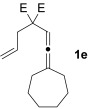 |
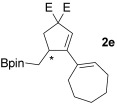 |
72 | 68 | 92 |
| 6 | 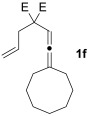 |
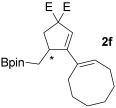 |
96 | 86 | 88 |
| 7 | 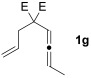 |
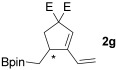 |
96 | 34 | 51 |
| 8 | 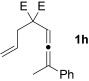 |
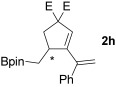 |
48 | 75 | 71 |
| 9 | 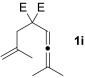 |
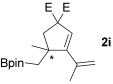 |
96 | 79 | 75 |
| 10 | 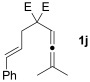 |
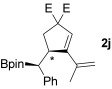 |
48 | 0 | –[d] |
| 11 | 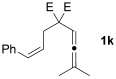 |
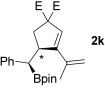 |
86 | 10 | 68 |
| 12 | 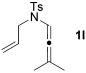 |
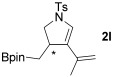 |
48 | 0 | –[d] |
| 13 | 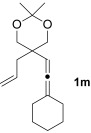 |
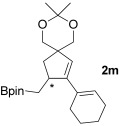 |
108 | 76 | 93 |
Reaction conditions: Enallene (0.2 mmol), Pd(OAc)2 (5 mol %), 4 a (10 mol %), B2pin2 (1.0 equiv), and BQ (1.5 equiv) in anhydrous m-xylene (1.0 mL) under argon at 13 °C.
Yields of isolated products after column chromatography.
Determined by HPLC on a chiral stationary phase.
Not determined. E=CO2Me, Ts=para-toluenesulfonyl.
