Figure 4.
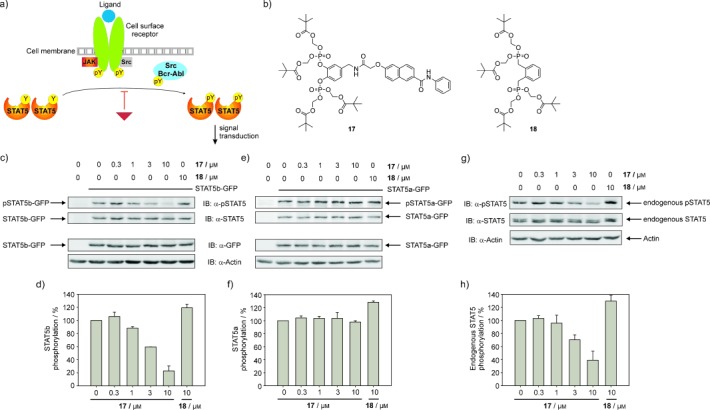
Selective inhibition of STAT5b tyrosine phosphorylation by 17, the pivaloyloxymethyl ester of 13. a) STAT signaling is induced by activated cell surface receptors and non-receptor tyrosine kinases. A small-molecule inhibitor of a STAT SH2 domain (the red triangle) inhibits STAT signaling by inhibiting STAT phosphorylation at the conserved tyrosine residue. The graphic is modified from the literature.[20] b) Structure of 17 and the negative control compound 18. c) Inhibition of STAT5b tyrosine phosphorylation by 17 in K562 cells transfected with STAT5b-GFP. A separate gel was run to confirm even transfection via the GFP tag. d) Quantification of the pSTAT5b-GFP bands, normalized against total STAT5b-GFP. Error bars represent the standard deviations from two independent experiments. e) Tyrosine phosphorylation of STAT5a in K562 cells transfected with STAT5a-GFP is not inhibited by 17. A separate gel was run to confirm even transfection via the GFP tag. f) Quantification of the pSTAT5a-GFP bands, normalized against total STAT5a-GFP. Error bars represent the standard deviations from two independent experiments. g) Effect of 17 and 18 on tyrosine phosphorylation of endogenous STAT5. h) Quantification of the endogenous pSTAT5 bands, normalized against total endogenous STAT5. Error bars represent the standard deviations from four independent experiments.
