Figure 1.
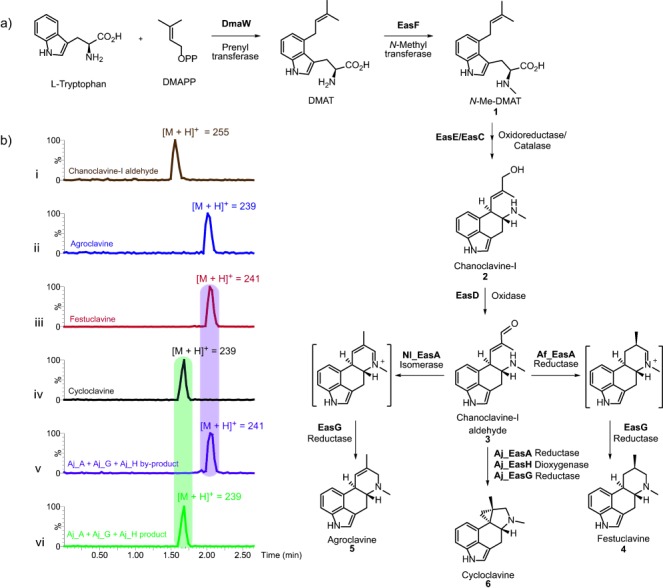
Ergot-alkaloid biosynthetic pathway. a) Biosynthesis of festuclavine (4), agroclavine (5), and cycloclavine (6) from l-tryptophan and dimethylallyl pyrophosphate (DMAPP). b) LC–MS chromatograms showing that EasA, EasG, and EasH are required to generate cycloclavine (6) from chanoclavine-I aldehyde (3). i–iv) Authentic standards of chanoclavine-I aldehyde (3; i), agroclavine (5; ii), festuclavine (4; iii), and cycloclavine (6, iv). v, vi) Reaction products from the incubation of Aj_EasA, Aj_EasG, and Aj_EasH with chanoclavine-I aldehyde (3) and cofactors: v) by-product, festuclavine (4); vi) predominant enzymatic product, cycloclavine (6).
