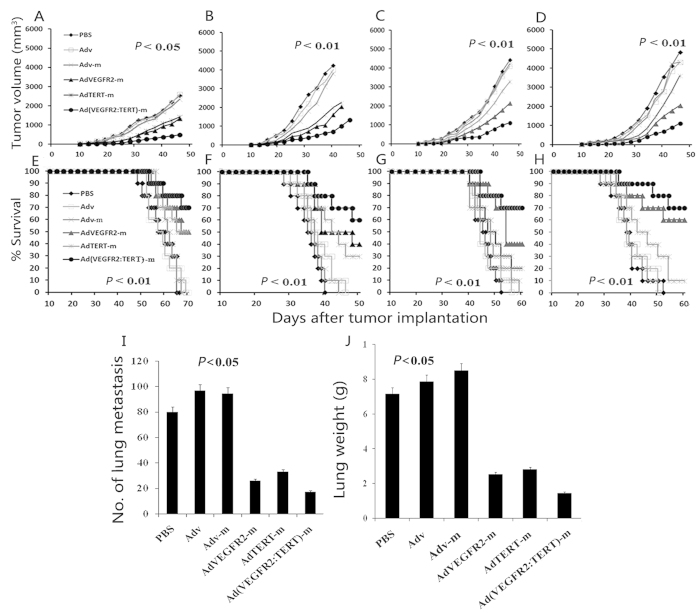
Figure 1. In vivo antitumour effects of AdVEGFR2-m and AdTERT-m combination therapy.
Mice were immunized with 1 × 108 PFU/100 μl of vaccine once a week for four weeks, and at one week after the fourth immunization they were challenged with 5 × 105 4T1 (A and E) and CT26 (B and F) cells, or they were first challenged subcutaneously with 5 × 105 4T1 (C and G) and CT26 (D and H) and then after seven days immunized with 1 × 108 PFU/100 μl of vaccine once a week for four weeks. There was a significant difference in tumour size between the combined AdVEGFR2-m and AdTERT-m group and control groups (A-D, p < 0.05, repeated measures ANOVA). The combined vaccine Ad(VEGFR2:TERT)-m also prolonged survival of mice compared with controls (E-H, p < 0.01, log-rank test). Subsequently, mice were immunized as described above and 5 × 105 LL/2 cells were injected into the tail vein seven days after the last immunization. Once the control mice appeared moribund (21 days after tumour injection), all mice were sacrificed and the resected lungs were weighed and assessed for metastatic nodules on the surface. The combined vaccine decreased the number of metastatic nodules (I) and alleviated tumour burden (J) (p < 0.05, one-way variance test). The results were expressed as the mean ± SD.