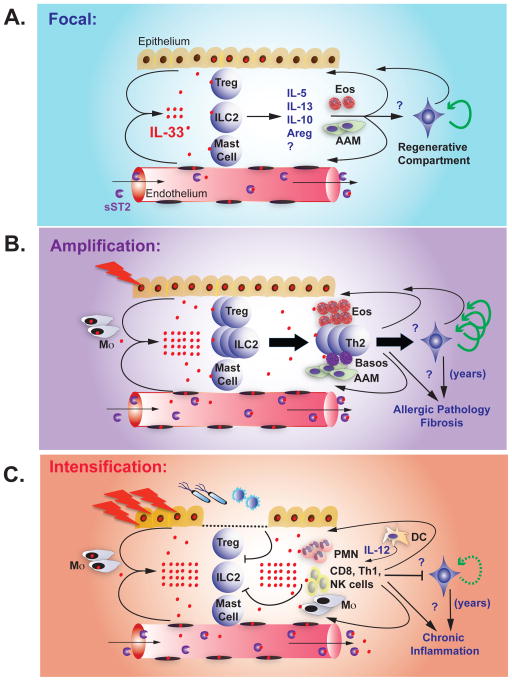
Figure 2. Progressive stages of IL-33-ST2.
A. Homeostasis. IL-33 is present in the nuclei of a subset of epithelial and endothelial cells at rest. Poorly understood signals promote IL-33 release or focal cellular necrosis. IL-33 acts on tissue-resident ST2-expressing ILC2, Treg, and possibly mast cells, inducing the production of IL-5, IL-13, IL-10, amphiregulin (Areg), and other signals that promote eosinophils (Eos) and alternatively activated macrophages (AAM). These cells and signals feed back on the tissue and may regulate remodeling and limit inflammation, in part by activating tissue progenitor cells. sST2 in the blood prevents systemic IL-33 effects.
B. Amplification. During tissue allergic insults and injury, epithelial and endothelial cells release IL-33, likely via necrosis, and IL-33 expression is further induced. Increased extracellular IL-33 activates and expands tissue ILC2 and Treg, and promotes recruitment and survival of additional immune cells (eosinophils, AAM, Th2 cells, basophils). These cells and signals feed back on the tissue to promote remodeling and limit inflammation, in part by activating tissue progenitor cells. In acute injury (infarction, tissue damage, helminth infection) these pathways help resolve injury and limit helminth infection. Chronic stimuli, such as allergens and repetitive tissue damage, lead to multiple cycles of IL-33 release that promote chronic allergic pathology, tissue fibrosis, increased Th2 cells, loss of Treg and other regulatory components, and increased tissue stores of IL-33. sST2 production is increased, but IL-33 concentrations may exceed blood buffering capacity in chronic damage and lead to systemic effects.
C. Conversion. Infectious or inflammatory triggers elicit tissue damage and epithelial breaches in the context of pathogen-associated molecular patterns. Pre-formed tissue IL-33 stores are released, likely via necrosis, and IL-33 is further induced in tissue cells. Inflammation and foreign antigen induces dendritic cell activation and IL-12 production, trafficking of inflammatory leukocytes from blood, and increased responsiveness to IL-33 through upregulation of ST2 on additional cell types. Activated inflammatory cells and cytokines, including IFN-γ, repress the Treg and type 2 immune response and facilitate killing of microbial and viral pathogens. In chronic inflammatory states, such as COPD and possibly autoimmune disease, IL-33 pools are increased and promotes repetitive cycles of IL-33 release, increasing tissue damage. sST2 production is increased, but IL-33 levels may exceed blood buffering capacity in chronic damage and lead to systemic effects.