Figure 6.
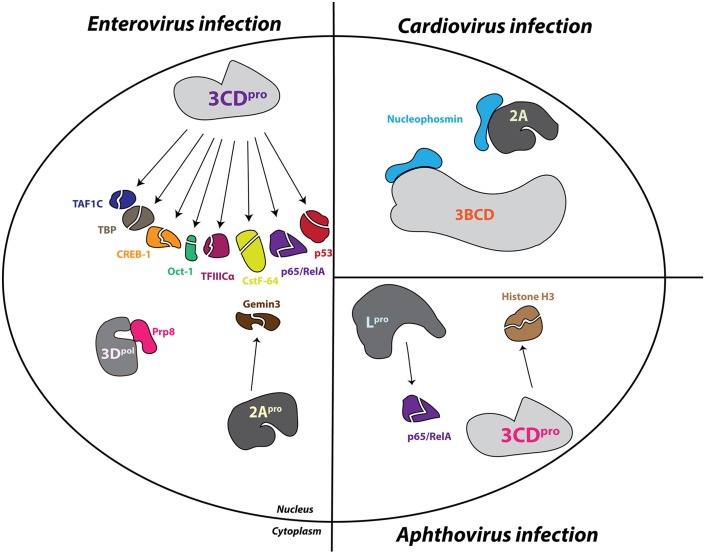
Picornavirus proteins enter the nucleus and alter nuclear-resident proteins. During enterovirus infections, the viral proteinase 3CD/3C (3CDpro) enters the nucleus and degrades TATA box-binding protein-associated factor RNA polymerase I subunit C (TAF 1C), leading to inhibition of RNA Polymerase I (Pol I) transcription. Pol III-driven transcription is also inhibited by 3CDpro, through cleavage of general transcription factor IIIC polypeptide 1 (TFIIICα). Pol II transcription, including cellular mRNA production, is terminated in enterovirus infected cells through cleavage of TATA-box-binding-protein 1 (TBP1), cyclic AMP-responsive element-binding protein 1 (CREB-1), and POU domain, class 2, transcription factor 1 (Oct-1) by 3CDpro. The transcription factors cellular tumor antigen p53 (p53), cleavage stimulation factor subunit 2 (CstF-64), and the NF-κB subunit p65/RelA (p65/RelA) are also degraded in a 3CDpro dependent manner during enterovirus infections. Furthermore, the enteroviral polymerase 3D (3Dpol) associates with the splicing factor pre-mRNA processing factor 8 (Prp8), causing dysregulation of splicing, and the 2A proteinase (2Apro) cleaves Probable ATP-dependent RNA helicase DDX20 (Gemin3). Cardiovirus infection causes the nuclear localization of both 2A and precursor protein 3BCD, both of which may associate with nucleophosmin. Infection with FMDV causes the cleavage of both Histone H3 as well as p65/RelA within the nucleus, following the entry of both L proteinase (Lpro) and 3CDpro.