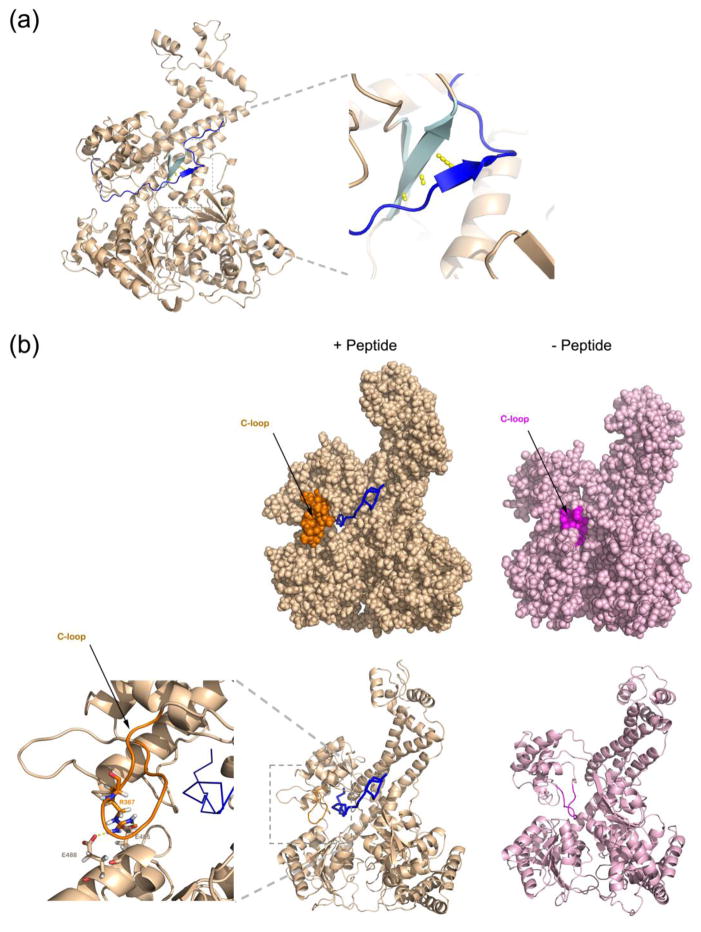
Figure 3. Molecular dynamics simulation of SecA with a peptide inside the clamp.
(a) A 30-amino acid peptide was placed inside the closed clamp of T. maritima SecA (see Figure S3a). Shown is the position of the main chain of the peptide (blue line) after 50 ns of the simulation run. A short segment of the peptide forms a β-strand (dark blue arrow) that interacts through hydrogen bonds (in yellow) with the β-sheet at the back of the clamp (cyan arrows). The right panel shows a magnified view of the augmented β-sheet.
(b) Peptide binding to the clamp moves the C-loop (residues 360-370) from the interior (right panels) towards the outside (left panels), where it interacts with NBD2. The C-loop in the two states is shown in magenta and orange, respectively. The upper panels show space-filling models, the lower ones show the secondary structure of the main chain. The left-most panel shows a magnified view of the interaction between the C-loop and NBD2 with the residues involved in stick presentation. The substrate peptide is in shown in ribbon presentation in blue.