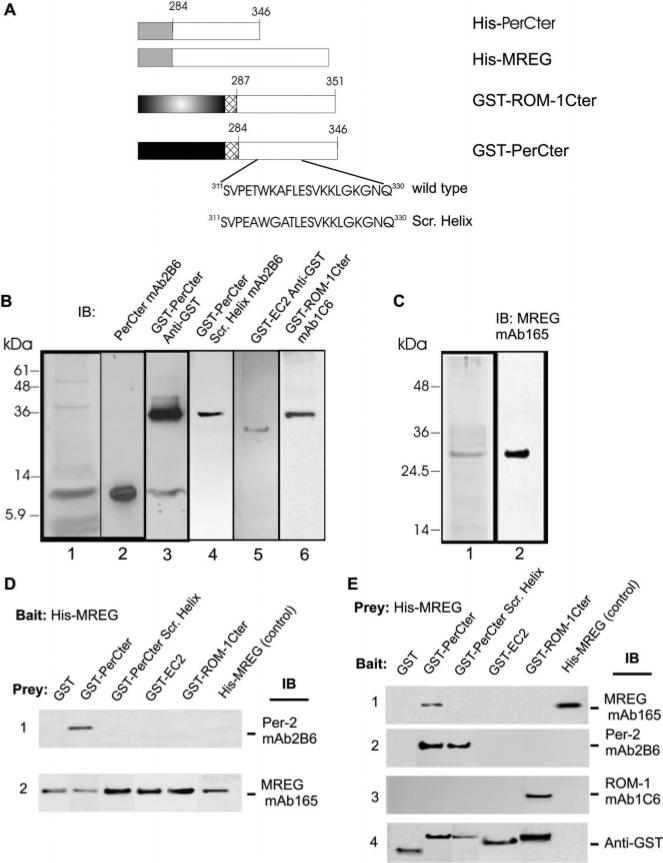
FIGURE 4.
Construction, expression, and interaction of the GST–PerCter construct with MREG. (A) Schematic diagram showing the structure of fusion proteins, hexahistidine-tagged peripherin-2 C-terminus (HisPerCter), hexahistidine-tagged MREG (His–MREG), and GST-tagged peripherin-2 C-terminus wild type and scrambled helix (GST–PerCter and GST–PerCterScr.Helix, respectively). (B) Coomasie blue-stained gel (lane 1) showing expression of purified PerCter and Western blot analysis of purified PerCter using mAb2B6 (lane 2) and GST–PerCter fusion protein using anti-GST (lane 3) as well as purified control GST–polypeptide constructs: GST–PerCterScr.Helix using mAb2B6 (lane 4), GST–EC2 using anti-GST antibody (lane 5), and GST–ROM-1Cter using mAb1C6 (lane 6). (C) Coomasie blue-stained gel of purified His–MREG (lane 1) and Western blot analysis of His–MREG fusion protein probed with anti-MREG mAb165 (lane 2). (D) His–MREG interacts with PerCter. The His–MREG fusion protein attached to the Ni2+ resin was incubated with purified GST–polypeptide constructs at 4 °C overnight as detailed in Methods. Interaction of MREG and the purified PerCter was verified by Western blot analysis with the anti-peripherin-2 mAb2B6 (lane 1). The binding of the bait to the Ni2+ resin was confirmed using anti-MREG mAb165 (lane 2). (E) The GST-PerCter fusion protein interacts with His–MREG. The GST fusion proteins attached to the glutathione resin were incubated with purified His–MREG at 4 °C overnight as detailed in Methods. Interaction of MREG and the purified PerCter was verified by Western blot analysis with the anti-MREG mAb165 (lane 1). The binding of the GST–polypeptide constructs to the glutathione resin was confirmed using mAb2B6 (lane 2), mAb1C6 (lane 3), and anti-GST antibody (lane 4).