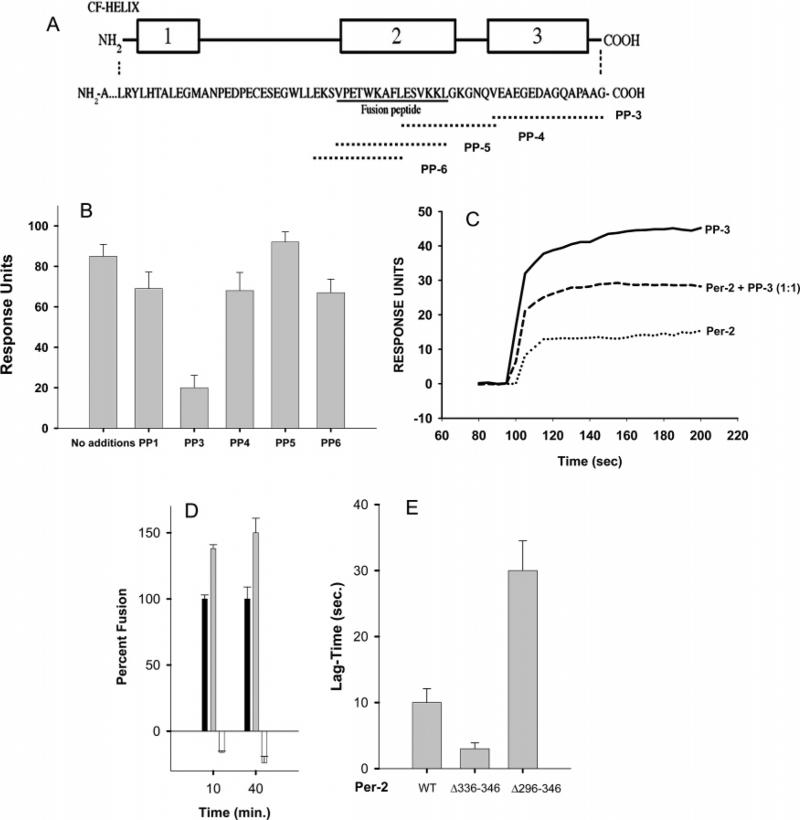
FIGURE 6.
Identification of a region of PerCter that binds MREG using peptide competition studies. (A) C-Terminal domain of bovine peripherin-2 corresponding to residues 289–346. Regions 1–3 represent α-helical domains as predicted by Chou–Fasman analysis (54). The series of overlapping peptides used in the competition studies is given. (B) Binding of PerCter to MREG is inhibited by PP-3, a peptide corresponding to the terminal 10 residues of peripherin-2. The binding of PerCter preincubated with the indicated peptides to immobilized MREG was followed using SPR as described in Methods. RU changes upon the addition of individual peptides are shown. Data represent three independent binding studies, each of which was carried out in duplicate. (C) Representative sensorgrams of PP-3, PerCter, and an equimolar mixture of PP-3 and PerCter, binding to immobilized MREG. Fusion of R18 plasma membrane with target membranes containing peripherin-2 mutants. (D) Fusion between R18PM and purified F18-labeled COS-7 cell membranes enriched with full-length FLAG-peripherin-2 (black), FLAG-per2Δ336–346 (gray), or FLAG-per2Δ296–346 (white) was assessed as an increase in R18 fluorescence over time at 37 °C as described in Methods. Data represent two independent experiments with fusion assays performed in triplicate. (E) Lag time of fusion between R18PM. The time prior to a linear increase in R18 fluorescence indicative of membrane fusion is plotted for the fusion reactions shown in panel D.