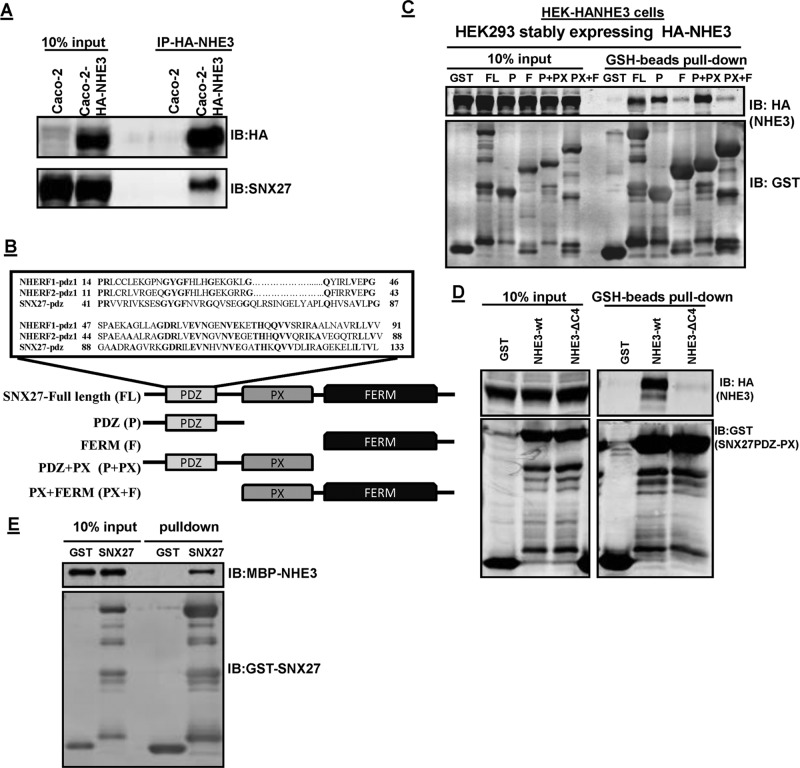
FIGURE 1:
SNX27 and NHE3 directly interact with each other. (A) Interaction between endogenous SNX27 and NHE3 in Caco-2-HA-NHE3 cells. NHE3 (HA-NHE3) was immunoprecipitated (IP) from total lysate of Caco-2BBe cells using anti-HA antibody. Immunoprecipitated samples were subjected to Western blot analysis (IB) and probed with HA and SNX27 antibodies. Representative result from three independent experiments with similar results. (B) Schematic of SNX27 PDZ domain organization showing the PDZ domain, the PX domain, and the FERM domain of SNX27. Expanded box shows sequence comparison of the SNX27 PDZ domain with the first PDZ domain of NHERF1 and NHERF2. Conserved residues in the three PDZ domains are shown in bold. (C) HEK-HA-NHE3 cell extracts (1 mg) were incubated with GST or GST-fusion proteins: 1 nmol; SNX27-FL (aa 1–539), PDZ (P; aa 1–156), FERM (F; aa 272–539), PDZ+PX (P+PX; aa 1–266), PX +FERM (PX+F; aa 158–539). The presence of NHE3 in GST pull downs was detected by Western blotting (top). Equal GST loading was verified by Western blotting for GST. Representative results from three independent experiments. (D) HEK-HA-NHE3 or HA-NHE3∆C4 cell extracts (1 mg) were incubated with GST or GST-fusion proteins (1 nmol; SNX27- PDZ+PX) and then subjected to pull down with GSH resin. The presence of NHE3 in GST pull downs was detected by Western blotting (top). Representative results from three independent experiments. (E) GST or GST-SNX27-FL was mixed with MBP-NHE3-C-term (aa 642–832) and then subjected to pull down with GSH resin. Samples were analyzed by Western blot (IB) with antibodies against MBP and GST. The experiment was repeated three times, and one representative result is shown.