FIGURE 2:
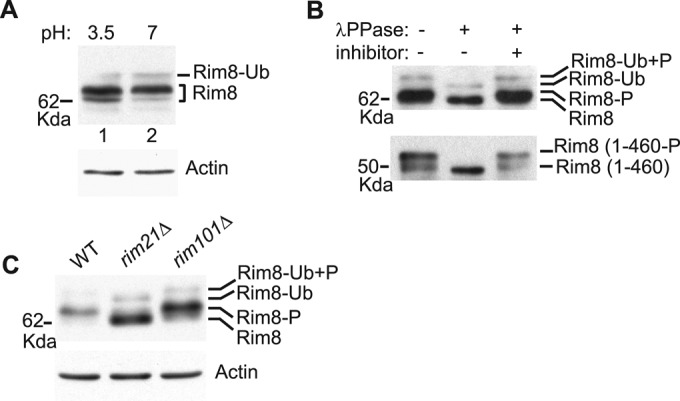
Phosphorylation of Rim8. (A) Effect of ambient pH on Rim8 electrophoretic mobility. Protein extracts from strain OVY247 (RIM8-3HA) prepared before and after a shift from pH 3.5 to 7 were prepared by glass bead disruption and immunoblotted with anti-HA (top) or anti-actin (bottom) antibodies. (B) Phosphatase assay. Y04414 (rim8∆) was transformed with pHA-Rim8 (top) or a C-terminal truncated derivative (residues 1–460; bottom), grown to mid log phase, and shifted to pH 7.5. Anti-HA–immunoprecipitated protein extracts were treated with λ phosphatase (λPPase) with or without phosphatase inhibitor and immunoblotted with anti-HA antibody. Positions of the different Rim8 forms (P, phosphorylated; Ub, ubiquitinated) are indicated. (C) Rim21-dependent phosphorylation of Rim8. OVY247 (RIM8-3HA), OVY270 (rim21∆ RIM8-3HA), and OVY271 (rim101∆ RIM8-3HA) were grown to mid log phase (final pH 3.5), and protein extracts were immunoblotted with anti-HA (top) or anti-actin (bottom) antibodies.
