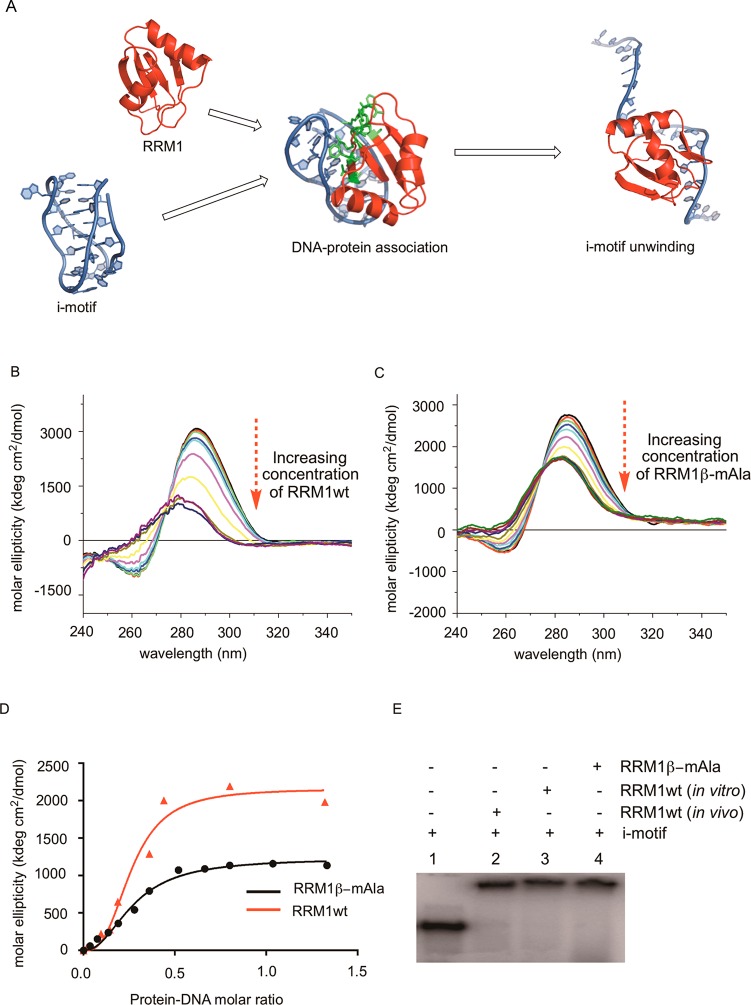
Figure 10.
Binding interaction between the i-motif DNA and RRM1 containing Ala or β-mAla (4) at position 35. (A) Schematic representation of i-motif and RRM1 binding resulting in the unwinding of i-motif DNA. (B) CD spectra of i-motif DNA upon its titration with RRM1wt until saturation. (C) Curves of normalized molar ellipticity ([θ]DNA − [θ]DNA + protein) at 286 nm as a function of protein-DNA molar ratio for RRM1wt and RRM1β-mAla. (D) Dissociation constant (Kd) for DNA protein binding in the presence of Ala or β-mAla at position 35. (E) Electrophoretic mobility shift assay (EMSA) with radiolabeled i-motif and RRM1wt derived from in vivo and in vitro expression, or RRM1β-mAla expressed in vitro.