Abstract
Background
Only limited evidence is available regarding the cytokine repertoire of effector T cells associated with peanut allergy, and how these responses relate to IgE antibodies to peanut components.
Objective
To interrogate T-cell effector cytokine populations induced by Ara h 1 and Ara h 2 among peanut allergic (PA) children in the context of IgE, and to evaluate their modulation during oral immunotherapy (OIT).
Methods
Peanut-reactive effector T cells were analyzed in conjunction with specific IgE profiles in PA children using intracellular staining and multiplex assay. Cytokine-expressing T cell subpopulations were visualized using SPICE.
Results
Ara h 2 dominated the antibody response to peanut as judged by prevalence and quantity among a cohort of children with IgE to peanut. High IgE (>15 kUA/L) was almost exclusively associated with dual sensitization to Ara h 1 and Ara h 2, and was age-independent. Among PA children, IL-4-biased responses to both major allergens were induced, regardless of whether IgE antibodies to Ara h 1 were present. Among subjects receiving OIT in whom high IgE was maintained, Th2 reactivity to peanut components persisted despite clinical desensitization and modulation of allergen-specific immune parameters including augmented specific IgG4 antibodies, Th1 skewing and enhanced IL-10. The complexity of cytokine-positive subpopulations within peanut-reactive IL-4+ and IFN-γ+ T cells was similar to that observed in those who received no OIT, but was modified with extended therapy. Nonetheless, high Foxp3 expression was a distinguishing feature of peanut-reactive IL-4+ T cells irrespective of OIT, and a correlate of their ability to secrete type 2 cytokines.
Conclusion
Though total numbers of peanut-reactive IL-4+ and IFN-γ+ T cells are modulated by OIT in highly allergic children, complex T-cell populations with pathogenic potential persist in the presence of recognized immune markers of successful immunotherapy. [ClinicalTrials.gov ID: NCT02350660]
Keywords: Peanut allergy, IgE, Ara h 1, Ara h 2, cytokines, Th1, Th2, oral immunotherapy
Introduction
Peanut allergy remains a major public health problem for school-age children in industrialized countries [1–3]. Currently, there is no cure, and conventional recommendations of strict avoidance place affected children at risk for anaphylaxis with accidental exposure [1, 3]. Detection of IgE antibodies to the major peanut allergen, Ara h 2, is the most important predictor of clinical reactivity upon peanut ingestion [4, 5]. Despite this, most T-cell studies have focused on the response to Ara h 1. There is evidence from a variety of experimental systems that peanut induces Th2-skewed responses among peanut allergic subjects [6–11]. A variety of mechanisms that implicate a pivotal role for dendritic cells (DCs) in driving Th2 responses to peanut have been identified. In the case of Ara h 1, this involves priming DCs via binding of glycan moieties to C-type lectin receptors [8, 9]. Similarly, in mice that are epicutaneously sensitized, Ara h 2 arms DCs for Th2 priming through the IL-33 pathway [12].
Since the first reports of successful desensitization for peanut allergy by oral immunotherapy (OIT) [13, 14], results of peanut OIT trials from multiple centers using different study designs have produced variable results [15]. It has been suggested that desensitization following OIT protects against the risk for IgE-mediated reactivity by virtue of Th2 suppression mediated by T regulatory cells [16]. However, definitive proof of T regulatory cell-mediated protection induced by OIT has been elusive, in part because identifying these cells in vitro is problematic owing to the lack of a reliable surface marker in humans [17]. On the other hand, the variable level of clinical protection observed in children who complete OIT trials raises the question of whether peanut-reactive pathogenic T cells are incompletely suppressed.
It was previously recognized that Th2 cells associated with peanut allergy are heterogeneous [11]. However, there is scant evidence of the T-cell cytokine repertoire induced by each of the major peanut allergens, and its relationship to IgE antibodies to peanut components. With these aspects in mind we sought to interrogate the T-cell cytokine repertoire induced by Ara h 1 and Ara h 2. Specifically, we aimed to explore T-cell characteristics in the context of high IgE in order to first understand how T-cell responses to Ara h 1 and Ara h 2 compare, and second, to assess the T-cell modulatory effects of OIT on complex T-cell populations induced by these allergens. Among children with high IgE who are nonetheless clinically desensitized, we identify multiple-cytokine-producing subtypes that are peanut-responsive and relatively stable. Further, we provide evidence that residual IL-4+ effectors that persist during OIT, while low in numbers, have the potential to readily re-activate in response to peanut. The clinical implications for treating peanut allergy are discussed.
Methods
Human Subjects
Ninety three children (ages 6 months to 19 years) were recruited through the University of Virginia Asthma and Allergic Diseases Clinic for IgE studies to peanut (see this journal’s Online Repository for clinical characteristics and Table S1). T-cell studies were performed in 29 children in whom blood volume was attainable (at least 5ml), including 21 peanut allergic (PA) and 8 non-peanut allergic subjects. Inclusion criteria for peanut allergy were: (1) IgE ab titer to peanut >0.35 kUA/L with a recent convincing history of clinical reactivity to peanut that occurred within 60 minutes of peanut ingestion or (2) IgE ab to peanut >0.35 kUA/L with a positive physician-supervised oral food challenge to peanut [18]. Non-peanut allergic children had no history of peanut allergy and maintained peanut in their regular diet. Incidence of peanut-induced anaphylaxis was determined by questionnaire. A history of atopic dermatitis, allergic rhinitis, asthma, eosinophilic esophagitis, and other food allergies was also documented (Table S1). Work was performed under protocols #15662 and #15098 approved by the University of Virginia Human Investigations Committee.
Oral Immunotherapy Regimen
Twenty children ages 4–18 years who met the above criteria for peanut allergy and had IgE antibody titers to peanut >15 kUA/L were enrolled in a pilot study of OIT (ClinicalTrials.gov ID: NCT02350660). Subjects were excluded if they had a history of severe peanut anaphylaxis (hypoxia, hypotension, or neurological compromise), moderate to severe persistent asthma, poorly-controlled atopic dermatitis, an inability to discontinue antihistamines for skin testing and food challenges, or a contraindication to epinephrine. Following a dose escalation phase, 17 subjects attained low dose maintenance (306mg of peanut flour) within 8 months (Fig. S1). Clinical desensitization to peanut was confirmed by open food challenge using a cumulative dose of 5 grams of peanut flour after 4 months on daily maintenance therapy. Assays for serum antibodies and T cell responses were performed on a subset of subjects using available blood obtained at baseline, and at 12–24 and 30 months after initiating treatment (Fig. S1). See Online Repository for further details.
Serum Antibody Assays
Specific IgE and IgG4 antibodies (ab) to whole peanut (f13) as well as Ara h 1 (f422) and Ara h 2 (f423) were measured by ImmunoCAP assay (Phadia US, Portage, MI) using the ImmunoCAP 250 system. Limits of detection for specific IgE and specific IgG4 assays were 0.35 kUA/L and 0.07 micrograms of antigen-specific antibody per milliliter (µgA/mL) respectively.
Flow Cytometry Antibodies and Other Reagents
See Methods in Online Repository for details.
Flow Cytometry Analysis of CD4+ T Cells
Freshly isolated PBMCs were cultured by established methods [19]. Briefly, PBMCs were plated in 24-well plates (1×106 cells/ml), and stimulated with Ara h 1 or Ara h 2 (20µg/ml) or Fel d 1 (10µg/ml). 20µg/ml was selected for stimulation with Ara h 1 and Ara h 2 and 10µg/ml for Fel d 1 based on equivalent maximal responses in dose response experiments using 10–50µg/ml. For some assays, PBMCs were labeled with CFSE (Invitrogen) prior to culture. On day 7, cells were restimulated with phorbol 12-myristate 13-acetate (PMA)(50ng/ml; Fisher Scientific) and 2µg/ml ionomycin (Invitrogen) in the presence of Brefeldin A (BD Biosciences, San Jose, CA) for 4 hours [19]. Cells were then stained for surface and intracellular markers and analyzed using an LSRII flow cytometer (BD Biosciences). Data was acquired using FACS Diva software (version 6.0, BD Biosciences) and analyzed using Flow Jo 9.6 (Tree Star). Dead cells were excluded by Aqua staining. Since CD4 is downregulated on T cells after activation with PMA and ionomycin [20, 21], live CD3+CD8− cells were analyzed in order to capture total CD4+ T cell events, after excluding monocytes and B cells. For all multicolor analyses, compensation controls (single stains, 1 for each fluorochrome) and gating controls (cells stained with all reagents minus 1) were included [22, 23].
Cytokine Assays
Culture supernatants were assayed for cytokines by cytometric bead assay (Millipore, Billerica, MA) using a Bioplex System (BioRad, Hercules, CA).
Statistical Analysis
Correlations between IgE ab levels to peanut and age or specific IgE ab, were analyzed by Spearman’s test. T-cell percentages were analyzed within or between groups using linear mixed models after log transformation of data. Linear mixed models were also used to analyze expression of specific markers (MFI) among different T cell subsets for each allergen, and to compare secreted cytokine levels pre- versus post-OIT. Secreted cytokines were analyzed within-group by the non-parametric Wilcoxon rank sign test for paired data and between groups by the 2 sample Wilcoxon rank sum test. A Bonferroni type I error rate of 0.05 was considered significant. Analysis of complex cytokine populations was performed using SPICE version 5.3, downloaded from http://exon.niaid.nih.gov. Comparison of T-cell population distributions was performed using a Student’s t test and a partial permutation test as previously described [24].
Results
Ara h 2 Dominates the Peanut IgE Profile in Children with Clinical Allergy
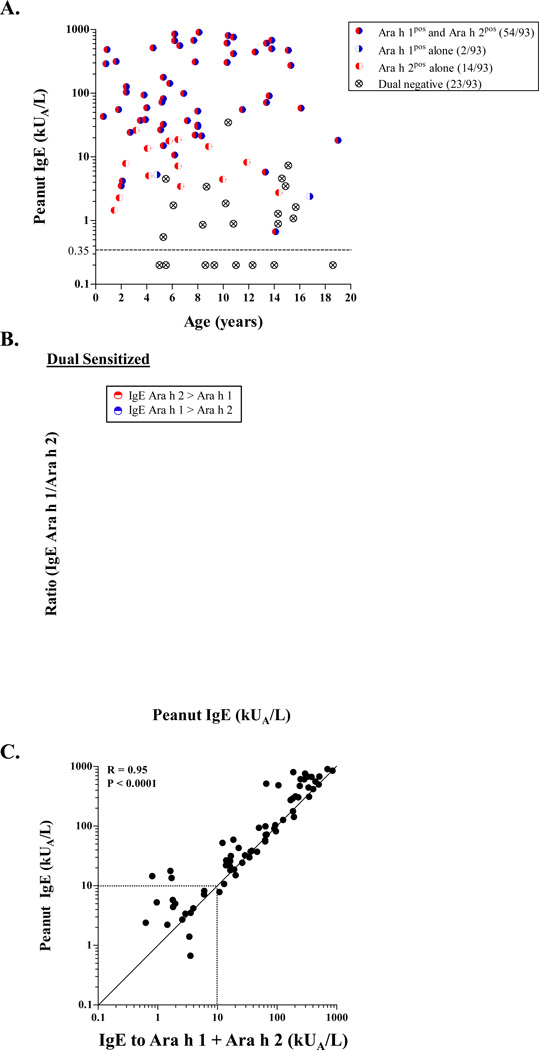
Among children with measurable IgE ab to peanut (n=85), eighty percent (54/85) were dual sensitized to both Ara h 1 and Ara h 2. This profile was associated with high IgE to peanut (≥30 kUA/L), and was age-independent (Fig. 1A). Within the dual sensitized group, IgE ab levels to Ara h 2 exceeded those for Ara h 1 (geometric mean = 41.1 kUA/L [95% CI: 26.2–64.6 kUA/L] versus 16.0 kUA/L [9.5–26.8 kUA/L] (p<0.001))(Fig. 1B). Single positivity to Ara h 2 was less common and restricted to those children with peanut IgE ≤20 IU/ml, whereas single positivity to Ara h 1 was scarce (Fig. 1A). Among those subjects who met inclusion criteria for clinical peanut allergy (49/85, peanut IgE = 61.1 kUA/L [36–104 kUA/L]), the majority were either dual sensitized or monosensitized to Ara h 2 (82% and 16% respectively)(Table S1). IgE ab to Ara h 1 and Ara h 2 accounted for the majority of the IgE response to peanut (r=0.95, p<0.0001)(Fig. 1C). These findings confirm segregation of discrete sensitization profiles according to IgE levels to peanut, and support an association between clinical allergy, high peanut IgE and dominant sensitization to Ara h 2.
Figure 1. Relationship Between IgE Antibodies to Peanut and its Major Allergens.
(A) Relationship between IgE to peanut and positivity for IgE ab to Ara h 1 and Ara h 2 according to age, among 93 atopic children. (B) Relationship between Ara h 2:Ara h 1 IgE ratio and IgE to peanut among 54 dual sensitized subjects. (C) Correlation between IgE to peanut and the sum of IgE ab to Ara h 1 and Ara h 2 among 69 children with IgE to peanut who had IgE ab to one or both of the major peanut allergens.
High IgE to Peanut is Linked to IL-4-Biased Responses to Major Peanut Allergens
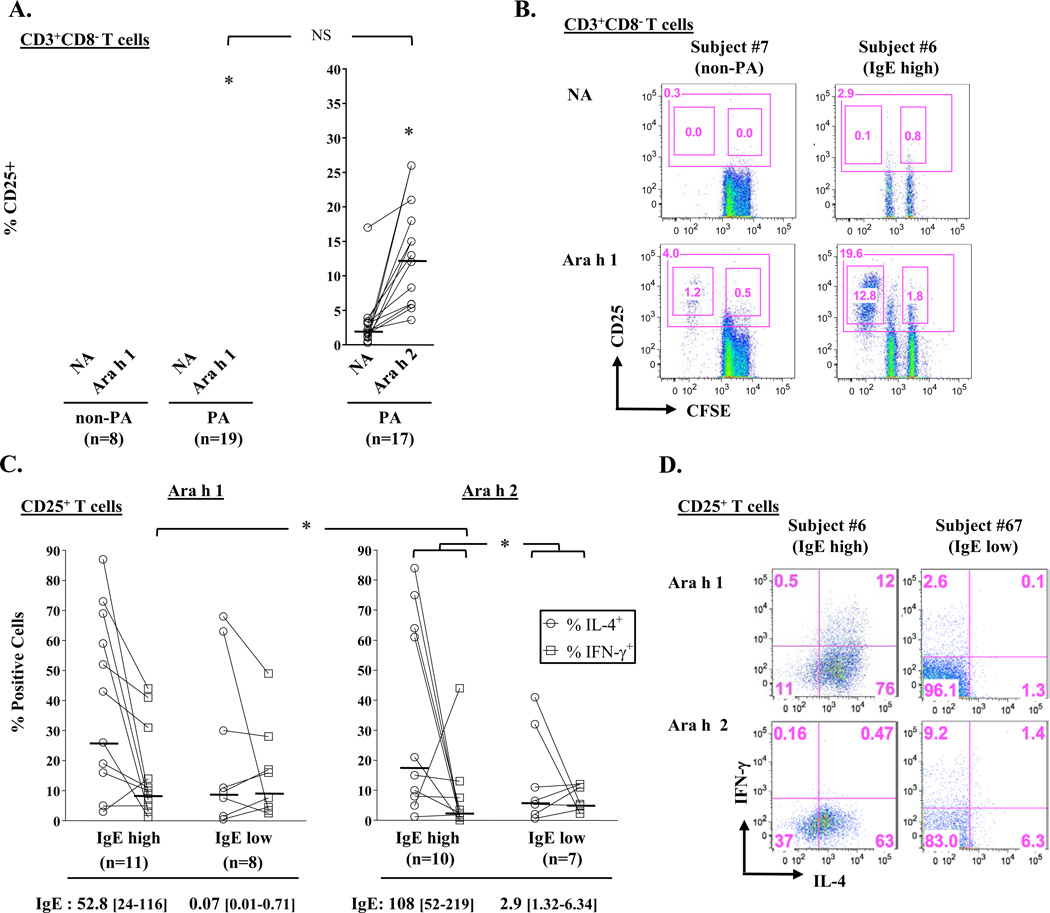
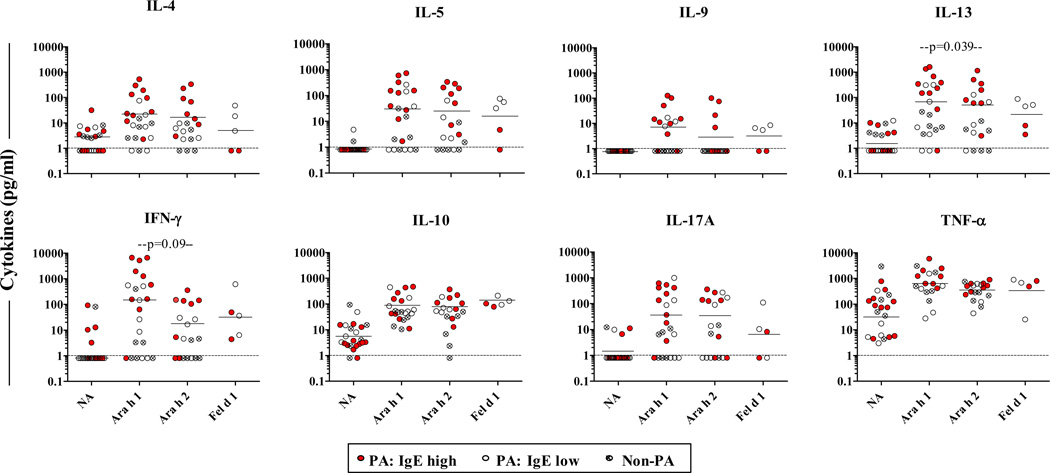
Next, we tested whether different IgE antibody profiles would reflect in the magnitude of T-cell activation induced by Ara h 1 and Ara h 2 in PBMC cultures from 19 children with clinical peanut allergy (14 dual sensitized and 5 mono-sensitized to Ara h 2)(Table 1)[25]. Ara h 1 induced T cell activation in all cultures, as judged by induction of proliferating CD25+CD4+ T cells, including in those from subjects who had no IgE ab to Ara h 1 (Figs. 2A & B). Moreover, T-cell activation induced by Ara h 1 was similar to that induced by Ara h 2, irrespective of serum antibody profile (Fig. 2A). By contrast, no T-cell activation was observed in non-allergic children. Thus, the presence of IgE ab to Ara h 2, and not Ara h 1, determines responsiveness to both major peanut allergens among PA children. When PA children were stratified into high IgE (>15 kUA/L) and low IgE (≤15 kUA/L) groups [18], increased Th2 skewing was evident among those with high IgE based on the ratio of activated IL-4+ T cells relative to IFN-γ+ T cells induced by Ara h 2 (ratio of geometric means = 7.42 versus 1.09 for IgE high versus IgE low group, p=0.034)(Figs. 2C & D). A similar IL-4-biased trend was observed in Ara h 1-stimulated cultures for PA children with high IgE; however, this effect was diminished (ratio of geometric means = 2.77 versus 0.90 for IgE high versus IgE low group, p=0.33) owing to increased IFN-γ+ T cells compared with Ara h 2 (p=0.014)(Fig. 2C). Both Ara h 1 and Ara h 2 induced secretion of high levels of the type 2 cytokines, IL-4, IL-5 and IL-13, in cultures from PA children, with IL-13 being the most abundant (Fig. 3). In accordance with T-cell staining for intracellular cytokines, the highest levels of secreted Th2 cytokines were observed for those cultures from children with high IgE. Further, Ara h 1 induced higher levels of IFN-γ versus Ara h 2, though this did not reach significance (Fig. 3). Together, these findings demonstrate augmented IL-4 responses to both major allergens in PA children sensitized to Ara h 2 in the context of high peanut IgE.
Table 1.
Characteristics of Children Included in T Cell Studies.
| Subject # | Age and gender1 |
Total IgE (IU/ml) |
IgE ab (kUA/L) | Clinical History | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Peanut | Ara h 1 | Ara h 2 | Cat | Peanut Reaction / Severity2 |
Other FA3 | AD4 | Asthma/ AR5 |
|||
| Non-PA | ||||||||||
| 3 | 18.6 (F) | 203 | <0.35 | ND | ND | <0.35 | NR | √ (TN) | √ | no |
| 7 | 8.6 (F) | 140 | <0.35 | ND | ND | <0.35 | NR | √ (CM) | √ | √ |
| 61 | 9.3 (F) | 9.3 | <0.35 | ND | ND | <0.35 | NR | no | √ | no |
| 64 | 4.9 (M) | 19.2 | <0.35 | ND | ND | <0.35 | NR | no | no | √ |
| 69 | 12.3 (F) | 5.2 | <0.35 | ND | ND | <0.35 | NR | no | no | no |
| 83 | 14 (F) | 240 | <0.35 | <0.35 | <0.35 | <0.35 | NR | no | no | √ |
| 70 | 5 (F) | 55.1 | <0.35 | <0.35 | <0.35 | <0.35 | NR | no | no | no |
| 2 | 5.3 (M) | 260 | 0.55 | <0.35 | <0.35 | 1.61 | NR | √ (EW) | √ | no |
| PA | ||||||||||
| 78 | 1.4 (F) | 39.3 | 1.4 | <0.35 | 3.2 | <0.35 | Urt/GI-2 | √ (EW, CM) | √ | no |
| 8 | 1.8 (F) | 418 | 2.22 | <0.35 | 1.1 | 1.33 | AE/Urt-1 | no | no | no |
| 67 | 2.1 (F) | 62.1 | 4.19 | 0.74 | 3.22 | <0.35 | AE/Urt-1 | √ (EW) | √ | no |
| 71 | 13.3 (M) | 620 | 5.78 | 0.98 | 0.81 | 186.2 | Urt/GI-2 | √ (EW, TN, SF) | √ | √ |
| 15 | 6.4 (M) | 825 | 7.14 | <0.35 | 5.64 | 7.33 | Urt/GI-2 | √ (TN) | √ | √ |
| 74 | 11.8 (F) | 1492 | 8.15 | <0.35 | 5.77 | 86.1 | AE/Urt-1 | √ (EW, TN) | √ | √ |
| 5 | 4.0 (F) | 347 | 13.5 | <0.35 | 1.36 | 3.97 | Urt-1 | √ (EW, CM) | √ | √ |
| 76 | 5.3 (F) | 321 | 15.0 | 7.99 | 12.2 | 4.26 | AE/Urt/GI-2 | √ (EW) | √ | no |
| 1* | 19.2 (F) | 114 | 18.2 | 6.49 | 9.96 | <0.35 | CV - 3 | √ (TN) | no | no |
| 81* | 8.3 (M) | 272 | 21.4 | 2.2 | 13.94 | 16.9 | AE/Urt - 1 | √ (TN, Se) | √ | √ |
| 46 | 7.2 (F) | 638 | 37.1 | 7.72 | 38.5 | 11.7 | AE/Urt-1 | √ (EW, Se) | √ | √ |
| 79 | 11.5 (M) | 267 | 55.5 | 46.5 | 16.8 | 5.15 | AE/Urt-1 | √ (TN, SF) | √ | √ |
| 60 | 13.6 (F) | 258 | 90.8 | 39.2 | 50.8 | 0.38 | AE/Urt-1 | √ (TN) | √ | no |
| 80* | 6.9 (M) | 254 | 99.1 | 15.6 | 48.2 | <0.35 | Urt/GI - 2 | √ (TN) | √ | √ |
| 11 | 15.3 (M) | 533 | 273 | 59.2 | 110 | <0.35 | Urt/GI-2 | no | no | √ |
| 23* | 10.8 (M) | 916 | 418 | 143.7 | 258.9 | 18.6 | Urt/Resp - 2 | √ (TN) | no | √ |
| 24* | 12.5 (F) | 1444 | 444 | 152.7 | 184.8 | 68.5 | CV/Resp - 3 | √ (TN) | √ | √ |
| 16* | 15.1 (F) | 1284 | 473 | 104.1 | 136.8 | 0.74 | CV/Resp - 3 | √ (TN) | √ | √ |
| 17* | 13.4 (F) | 1222 | 609 | 189.9 | 96.6 | 22.8 | Urt/GI - 2 | √ (TN) | √ | √ |
| 6 | 7.7 (M) | 1478 | 675 | 130 | 197 | 4.16 | Urt/GI-2 | √ (EW, So) | √ | √ |
| 40 | 13.8 (M) | >5000 | 680 | 29.2 | 483 | 68.5 | Urt/GI-2 | √ (CM, TN) | √ | no |
Age in years;
History of allergic symptoms within 1 hour of peanut ingestion, graded in accordance with severity classification of Brown [25]; NR, no reaction; AE, angioedema; CV, cardiovascular; Urt, urticaria; GI, gastrointestinal; Resp, respiratory
History of other food allergies (TN, tree nuts; EW, egg white; CM, cow’s milk; SF, shellfish; Se, sesame; So, soy);
History of atopic dermatitis;
History of asthma and/or allergic rhinitis.
Children who received peanut OIT – values are those obtained before starting OIT. Shaded rows denote PA children with high IgE.
M, male; F, female; ND, not determined.
Figure 2. High IgE to Peanut is Linked to IL-4-Biased T-Cell Responses.
(A) Percentage of CD25+CD4+ T cells in Ara h 1- and Ara h 2-stimulated PBMC cultures in PA children and non-peanut-allergic (non-PA) controls. Colored circles denote subjects monosensitized to Ara h 2 and dashed lines denote cut-off for T-cell activation. *p<0.001 for stimulated versus non-stimulated conditions. NA, no antigen. (B) Representative scatter plots. Large gate denotes total CD25+ cells and small gates denote CFSEdim (dividing) and CFSEhi (non-dividing) cells. Values represent cell percentages. (C) Percentage of IL-4+ and IFN-γ+ T cells induced by peanut allergens in cultures from “IgE high” (>15 kUA/L) and “IgE low” (≤15 kUA/L) PA children. *p≤0.034. IgE values are geometric means [95% CI] for each component. (D) Representative scatter plots showing intracellular staining for IL-4 and IFN-γ within CD25+ T cells. Horizontal bars denote geometric means. NS, not significant.
Figure 3. Secreted Cytokines Induced by Ara h 1 and Ara h 2.
Secreted cytokines in cultures from PA children (Ara h 1: n=17; Ara h 2: n=13) and non-PA controls (n=6). Data is also shown for Fel d 1-stimulated cultures from peanut/cat allergic children. Horizontal bars denote geometric means for all PA children. P values shown are for paired analyses on 13 PA children. For Ara h 1 and Ara h 2 versus no antigen, p≤0.007 for all cytokines in PA group and p>0.5 for all cytokines in non-PA group, except for TNF-α (p=0.043 for Ara h 1). Dashed lines denote the limit of sensitivity of the assay (1pg/ml). NA, no antigen.
Peanut-Reactive T Cells are Present Following OIT
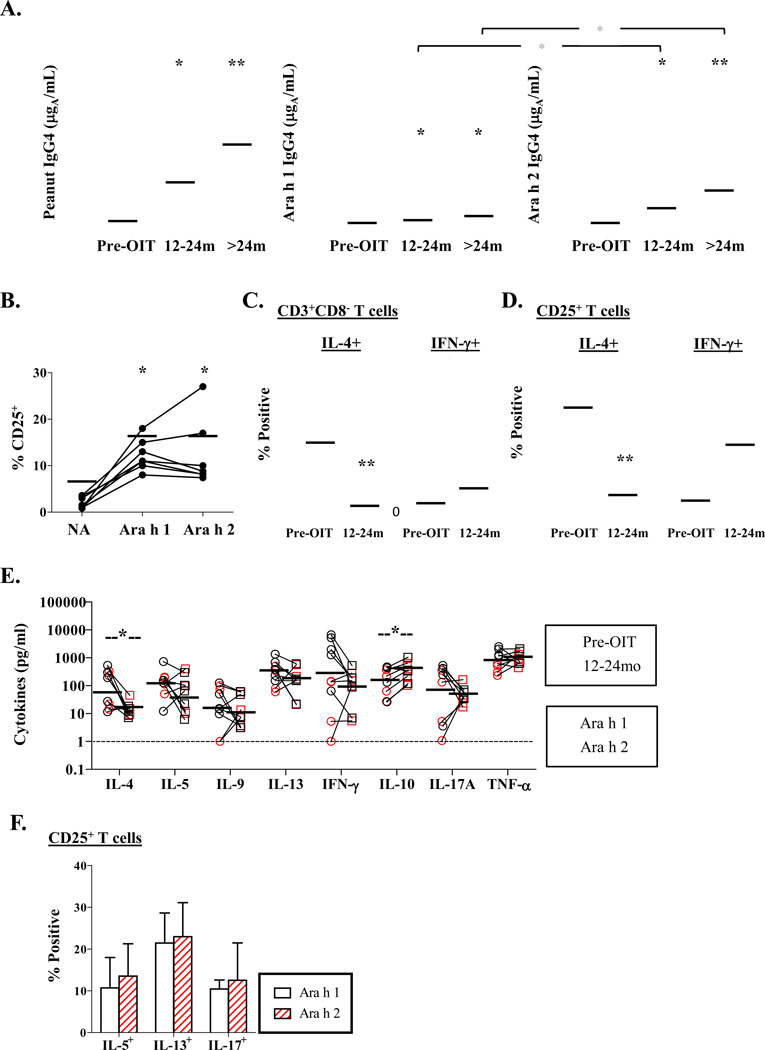
Participation of 20 PA children with high IgE to peanut in a pilot study of OIT (Fig. S1) allowed us to test for the presence of residual effector T cells that might undermine the beneficial effects of immunotherapy. Thirteen of 17 participants maintained high IgE to peanut (>15kUA/L) after 12–24 months (median 17 months) of treatment (Fig. S2). T-cell responses were analyzed among 7 of these children, all of whom remained on maintenance therapy (300–600mg). All children were desensitized to peanut based on open food challenge after 4 months of maintenance therapy. Peanut IgG4 ab were higher at 12–24 months as compared with baseline, and levels were higher for Ara h 2 as compared with Ara h 1 (p<0.05)(Fig. 4A). Both peanut allergens induced T cell activation among OIT children at similar levels to those observed for PA children who did not receive OIT (compare Fig. 4B with Fig. 2A, p>0.05). However, the IL-4-dominated profile was reversed following OIT. Specifically, the ratio of total numbers of peanut-reactive IL-4+ to IFN-γ+ cells was markedly reduced (ratio of geometric means = 15.4 versus 0.53), as well as the ratio of IL-4+ to IFN-γ+ cells within the activated subset (ratio of geometric means = 15.6 versus 0.53) after OIT (p<0.01)(Figs. 4C & D respectively). Though secretion of IL-4 in response to both Ara h 1 and Ara h 2 was decreased during OIT (p=0.04), levels of IL-4 and IL-13 remained elevated, despite increased secretion of IL-10 (p=0.05) (compare Fig 4E with basal secretion levels in Fig. 3). Low numbers of IL-10+ T cells were identified in cultures from PA children with high IgE, regardless of OIT, suggesting that non-T cells were the primary source of IL-10 following OIT (Fig. S3). Further analysis of peanut-reactive T cells by intracellular staining confirmed the presence of IL-5+, IL-13+ and IL-17+ T cells following OIT at frequencies corresponding to the profile of secreted cytokines (Figs. 4E & F). Taken together, these findings provide evidence of residual effector T-cell activation following peanut OIT.
Figure 4. Peanut-Reactive T Cells are Present Following OIT.
(A) Serum IgG4 antibody levels to peanut before and after reaching maintenance dose on OIT (n=17). *p<0.05 versus before OIT. Red symbols denote 7 children with persistent high IgE (>15 kUA/L) who were selected for T cell studies. Horizontal bars are geometric means. (B) Percentage of CD25+CD4+ T cells induced by peanut allergens in cultures from 7 children with high IgE who received 12–24 months OIT. *p<0.05, **p<0.01. (C) Percentage of CD25+IL-4+ and CD25+IFN-γ+ T cells within the CD3+CD8- subset, and (D) percentage of IL-4+ and IFN-γ+ T cells within the CD25+ subset induced by major peanut allergens among 5 children with high IgE who received 12–24 months OIT. Combined data is shown for Ara h 1 and Ara h 2 (black and red symbols respectively). **p<0.01. (E) Change in secreted cytokines induced by peanut allergens after 12–24 months OIT. *p≤0.05. (F) Percentage of IL-5+, IL-13+ and IL-17A+ T cells induced by peanut allergens within the CD25+ subset after 12–24 months OIT (mean ± SD, n=5).
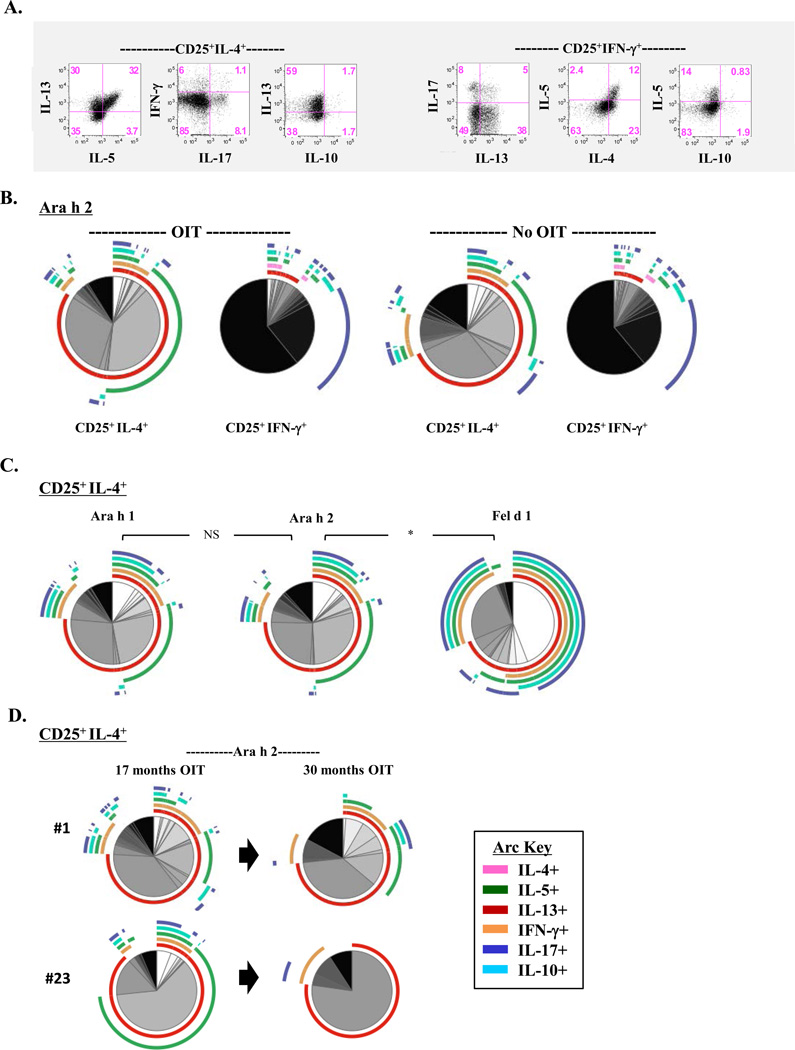
Multiple-Cytokine-Producing Effector T Cells Persist Following OIT
Persistence of high IgE to peanut among children undergoing OIT, coupled with residual secretion of effector T-cell cytokines suggested the persistence of complex T-cell types, despite a decrease in the absolute number of IL-4+ cells. Using conventional biaxial analyses, we confirmed the presence of peanut-reactive T cells within IL-4+ and IFN-γ+ subsets that secreted multiple cytokines following OIT (Fig. 5A). To further interrogate the quality of the T-cell cytokine repertoire following OIT, we generated a visual representation of all combinations of cytokine-producing cells within peanut-reactive IL-4+ and IFN-γ+ subsets. Notably, the profile of IL-4+ and IFN-γ+ T-cell subpopulations induced by Ara h 1 and Ara h 2 was not significantly different between PA children who received OIT and those with high peanut IgE who did not (Figs. 5B & S4A). Within the IL-4+ subset, cells predominantly expressed IL-13, with or without IL-5, with few cells expressing IL-4 alone, or else all 6 cytokines (IL-4, IL-5, IL-10, IL-13, IFN-γ and IL-17A). By contrast, IFN-γ+ cells predominantly expressed IFN-γ alone, though co-expression of IL-17A was also prominent (Fig. 5B). The proportion of IFN-γ+ cells that co-expressed Th2 cytokines comprised a minor subset, regardless of treatment status. Whereas the repertoire of cytokine-positive T cells within the IL-4+ subset was almost identical for Ara h 1 and Ara h 2 following 12–24 months OIT, this was distinct from that induced by Fel d 1 among 4 children who were sensitized to both peanut and cat (p=0.03)(Fig. 5C). Specifically, whereas IL-4+ T cells induced by Fel d 1 co-expressed IFN-γ, IL-17A, and IL-10, those induced by peanut allergens displayed a predominant Th2-like signature. Sustained low dose maintenance therapy for 30 months was associated with reduced complexity of the IL-4+ population, and loss of subpopulations expressing all 6 cytokines (Figs. 5D & S4B). Collectively, these findings show persistence of complex T-cell subpopulations within peanut-reactive IL-4+ and IFN-γ+ subsets during OIT.
Figure 5. Multiple-Cytokine-Producing Effector T Cells Persist Following OIT.
(A) Representative scatter plots from a child who received 17 months OIT, showing cytokine expression within IL-4+ and IFN-γ+ T-cells induced by Ara h 2. (B) Pie charts comparing the distribution of T cell subpopulations within IL-4+ and IFN-γ+ T-cell subsets induced by Ara h 2 in OIT (n=7) and non-OIT (n=5) groups. (C) Comparison of T-cell subpopulations with IL-4+ T-cell subsets induced by Ara h 1, Ara h 2, and Fel d 1 among 4 OIT subjects co-sensitized to peanut and cat. *p=0.03. (D) Effect of increased duration of OIT on the distribution of T-cell subpopulations within Ara h 2-induced IL-4+ T cells in cultures from 2 children.
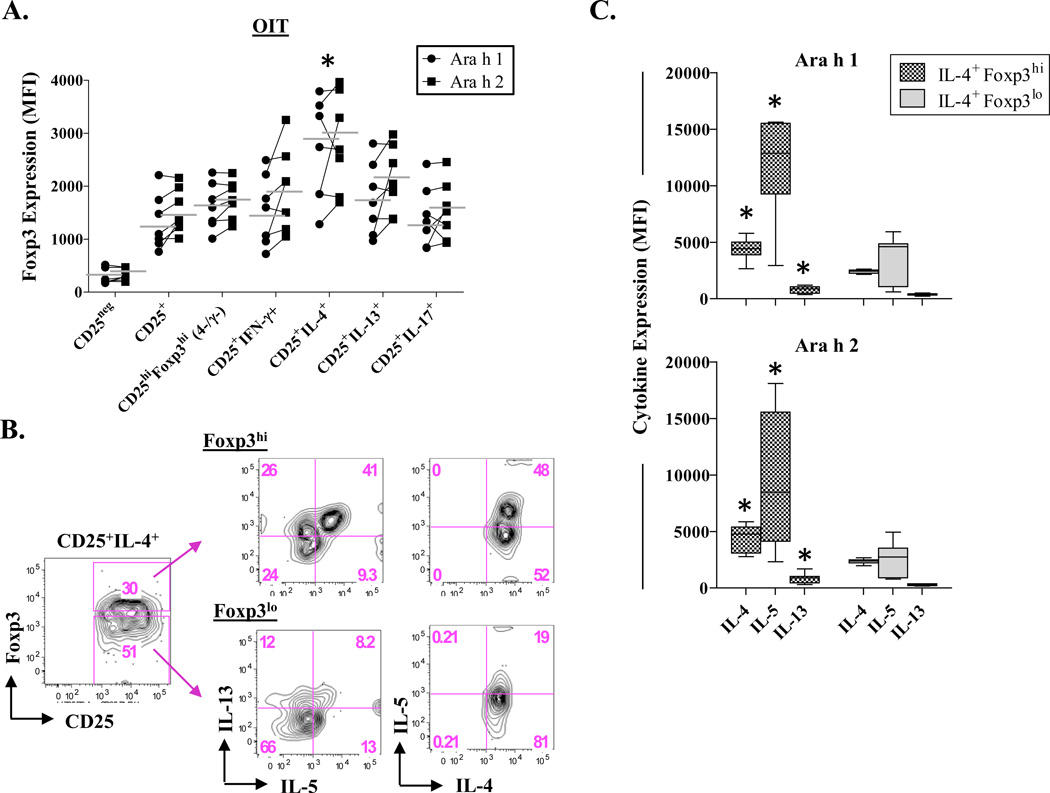
Residual IL-4+Foxp3hi T Cells Secrete Th2 Cytokines Following OIT
In addition to expressing CD25, activated effector T cells in humans express the transcription factor, Foxp3 [26–28]. To further assess the functional properties of residual peanut-reactive IL-4+ T cells following OIT, we compared Foxp3 expression levels across cytokine-positive T-cell subsets. After culture with peanut allergens, IL-4+ T cells expressed higher Foxp3 as compared with IL-13+, IFN-γ+ and IL-17A+ T cell types, irrespective of the allergen (p≤0.01). In addition, levels were higher than for cells with a T regulatory phenotype (CD25hiFoxp3hi)(p=0.05)(Fig. 6A). Notably, Foxp3 profiles were the same as those for PA children who did not receive OIT (Fig. S5). Among OIT children, analysis of those IL-4+ T cells expressing the highest levels of Foxp3 revealed higher expression of IL-4, IL-5 and IL-13 as compared with their Foxp3lo counterparts (p≤0.008)(Figs. 6B & C). Thus, high Foxp3 expression is a distinguishing feature of peanut-reactive IL-4+ T cells irrespective of OIT, and a correlate of their ability to secrete Th2 cytokines.
Figure 6. Residual IL-4+Foxp3hi T Cells Secrete Th2 Cytokines Following OIT.
Foxp3 expression profiles were analyzed in cultures from 7 children who received 12–24 months OIT. (A) Mean fluorescence intensity (MFI) of Foxp3 expression within T cell subtypes induced by peanut allergens. *p≤0.05 for IL-4+ T cells versus all other subsets. (B) Representative data showing Th2 cytokine expression in Foxp3hi and Foxp3lo IL-4+ T cells induced by Ara h 1. (C) Th2 cytokine expression in Foxp3hiIL-4+ and Foxp3loIL-4+ T cells (mean ± SD). *p≤0.008.
Discussion
In the present study, characterization of IgE responses to major peanut allergens among a cohort of atopic children provided context for the evaluation of peanut-reactive effector T cells among PA children, including those with persistent high IgE while on maintenance OIT. Ara h 2 dominated the IgE response based on both prevalence and quantitative measures. In addition, whereas low IgE to peanut was associated with monosensitization to Ara h 2, high IgE was almost exclusively associated with dual sensitization; however, these features were age-independent. Marked IL-4-biased responses to both major allergens segregated with high IgE to peanut. Though Th2 skewing as judged by the “balance” between peanut-reactive IL-4+ and IFN-γ+ cells was most pronounced for Ara h 2, the overall makeup of the T-cell cytokine repertoire induced by both major peanut allergens was similar, irrespective of whether IgE to Ara h 1 was present. Each allergen induced secretion of high levels of Th2 cytokines, including IL-4, particularly in cultures from children with high IgE. This feature was allergen-specific, based on discrepant results for Fel d 1 among those who were dual-sensitized to peanut and cat. Detection of such high levels of secreted IL-4 has not, to our knowledge, been reported previously using short-term PBMC cultures stimulated with either food or inhalant allergens [29, 30]. Thus, both major peanut allergens promote a uniquely robust Th2 response. Together, these findings support the view that Ara h 2 is a more potent allergen than Ara h 1, and that T-cell reactivity to Ara h 1 likely occurs through intermolecular epitope spreading [31].
All subjects participating in our pilot OIT program had IgE to peanut >15KUA/L prior to initiating treatment. Four subjects experienced a decrease in peanut IgE to levels <15kUA/L and had evidence of sustained unresponsiveness as judged by their ability to consume peanut without allergic symptoms after treatment was ceased. We chose to explore T-cell cytokines in more detail among those in whom high IgE levels were maintained, despite a rise in IgG4 to peanut, in order to better understand whether this reflected a transitional immune state. All of these subjects were clinically desensitized based on increased threshold of clinical reactivity to peanut in the face of continued OIT [32]. Analysis of peanut-reactive T-cells among these subjects revealed a “shift” from an IL-4- to an IFN-γ-dominated response to both Ara h 1 and Ara h 2 in these subjects. However, several features of the immune response indicated that residual pathogenic Th2 cells persist following 12–24 months of OIT. These included: (1) No significant reduction in serum IgE levels to peanut despite increased production of IgG4 antibodies and evidence of clinical desensitization; (2) Sustained peanut T-cell reactivity as judged by induction of CD25 and secretion of Th2 cytokines; (3) Detection of residual IL-4+ T cells displaying the same order of Th2 cytokine complexity as those present among PA children who did not receive OIT; (4) Lack of co-expression of IL-10) and segregation of high Foxp3 expression with augmented Th2 cytokine production within residual IL-4+ T cells. This latter observation is particularly important given that Foxp3 expression is known to be increased upon activation of effector T cells in humans [26–28]. Thus, we speculate that residual IL-4+ T cells that persist despite clinical desensitization, are poised to re-activate upon exposure to peanut. Such cells, while low in numbers, could serve to undermine the durability of nonresponsiveness to peanut. From a clinical perspective, both the dosage of allergen administered and the duration of treatment are likely to be key factors in determining whether pathogenic T cells are eliminated, or else completely suppressed, in vivo. Using intracellular cytokine staining, we report for the first time a reversal of allergen-specific Th2 skewing during peanut OIT. This could be explained by a variety of mechanisms including selective apoptosis of Th2 cells, suppression of Th2 cells, and conversion of Th2 to Th1 effectors or T regulatory cells, as well as induction or expansion of Th1 cells [33–35]. By dissecting the T-cell cytokine repertoire induced by both major allergens, we observed similar profiles of cytokine-expressing subpopulations within both IL-4+ and IFN-γ+ subsets, regardless of treatment. Notably the proportion of T cells co-expressing Th1 and Th2 cytokines was similar for OIT and non-OIT groups, thereby arguing against Th2 to Th1 conversion during treatment. Instead, our findings favor the notion of a selective reduction in Th2 cells coupled with expansion of Th1 cells. Among those who received OIT, the profile of IL-4+ T cells was more Th2-like for peanut- versus cat-reactive T cells in subjects sensitized to both allergen sources. Thus, the peanut-reactive Th2-like repertoire of cells that persists following OIT appears to be allergen-specific. Interestingly, with increased duration of treatment, the complexity of peanut-reactive IL-4+ T cells diminished based on disappearance of populations expressing all 5 cytokines tested. Thus, T cells continue to be modulated with extended therapy. These studies, which were performed in a small sample size, highlight the need for further studies in larger numbers of peanut allergic children in order to better understand T-cell mechanisms of OIT.
Our pilot OIT study used a maintenance dose of 300–600mg that falls within the range of that previously shown to attain clinical desensitization [13]. This dose, which was lower than that used in other clinical trials [16, 36, 37], was selected to improve compliance with the tolerability of daily peanut dosing for long-term adherence. Consistent with previous OIT studies, we observed marked increases in serum IgG4 antibodies to peanut that were most pronounced for Ara h 2 [13, 38, 39]. To date, elevated IgG4 is arguably the most reliable immune marker of desensitization. However, it is not known what levels are necessary to confer protection, or indeed whether peanut-specific IgG4 exerts functional “blocking” activity analogous to that associated with conventional immunotherapy [40, 41]. Recent work suggests that IgG induced during OIT can suppress peanut-induced basophil activation via FcγRIIb [42]. Whether this phenomenon impacts T-cell responses remains to be elucidated.
Future studies aimed at examining the evolution of complex effector T-cell populations are warranted in a larger group of children in order to identify the appropriate window for intervention. Our work supports the view that preventive strategies would need to be implemented very early in life, given that high IgE to peanut coupled with dual sensitization to major allergens is evident soon after birth. The failure for OIT to completely suppress T-cell responses to peanut allergens among PA children with high IgE who achieve maintenance dose raises questions about the durability of clinical desensitization and the risks posed to treated children who cease regular peanut ingestion.
Supplementary Material
Acknowledgments
The authors thank: Joanne Lannigan, MS and Michael Solga, MS (University of Virginia) for assistance with flow cytometry.
Funding Sources: This work was supported by the following grants from NIH/NIAID: R01 AI-052196 (J. Woodfolk) and U19 AI-070364 (Proj. 2, J. Woodfolk); and by an AAAAI/Food Allergy Initiative Howard Gittis Memorial Fellowship Award (J. Wisniewski).
J.A. Woodfolk receives research funds from NIH/NIAID and NIH/NIAMS. T.A. Platts-Mills and P.W. Heymann receive research funds from NIH/NIAID.
Abbreviations
- Ab
antibody
- DC
dendritic cell
- OIT
oral immunotherapy
- PA
peanut allergic
Footnotes
Conflicts of Interest
All other authors declared no conflicts of interest.
References
- 1.Fleischer DM, Perry TT, Atkins D, Wood RA, Burks AW, Jones SM, Henning AK, Stablein D, Sampson HA, Sicherer SH. Allergic reactions to foods in preschool-aged children in a prospective observational food allergy study. Pediatrics. 2012;130:e25–e32. doi: 10.1542/peds.2011-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sicherer SH. Epidemiology of food allergy. J Allergy Clin Immunol. 2011;127:594–602. doi: 10.1016/j.jaci.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 3.Vetander M, Helander D, Flodstrom C, Ostblom E, Alfven T, Ly DH, Hedlin G, Lilja G, Nilsson C, Wickman M. Anaphylaxis and reactions to foods in children--a population-based case study of emergency department visits. Clin Exp Allergy. 2012;42:568–577. doi: 10.1111/j.1365-2222.2011.03954.x. [DOI] [PubMed] [Google Scholar]
- 4.Dang TD, Tang M, Choo S, Licciardi PV, Koplin JJ, Martin PE, Tan T, Gurrin LC, Ponsonby AL, Tey D, Robinson M, Dharmage SC, Allen KJ. Increasing the accuracy of peanut allergy diagnosis by using Ara h 2. J Allergy Clin Immunol. 2012;129:1056–1063. doi: 10.1016/j.jaci.2012.01.056. [DOI] [PubMed] [Google Scholar]
- 5.Nicolaou N, Poorafshar M, Murray C, Simpson A, Winell H, Kerry G, Harlin A, Woodcock A, Ahlstedt S, Custovic A. Allergy or tolerance in children sensitized to peanut: prevalence and differentiation using component-resolved diagnostics. J Allergy Clin Immunol. 2010;125:191–197. e1–e13. doi: 10.1016/j.jaci.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Turcanu V, Maleki SJ, Lack G. Characterization of lymphocyte responses to peanuts in normal children, peanut-allergic children, and allergic children who acquired tolerance to peanuts. J Clin Invest. 2003;111:1065–1072. doi: 10.1172/JCI16142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeLong JH, Simpson KH, Wambre E, James EA, Robinson D, Kwok WW. Ara h 1-reactive T cells in individuals with peanut allergy. J Allergy Clin Immunol. 2011;127:1211–1218. e3. doi: 10.1016/j.jaci.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shreffler WG, Castro RR, Kucuk ZY, Charlop-Powers Z, Grishina G, Yoo S, Burks AW, Sampson HA. The major glycoprotein allergen from Arachis hypogaea, Ara h 1, is a ligand of dendritic cell-specific ICAM-grabbing nonintegrin and acts as a Th2 adjuvant in vitro. J Immunol. 2006;177:3677–3685. doi: 10.4049/jimmunol.177.6.3677. [DOI] [PubMed] [Google Scholar]
- 9.Royer PJ, Emara M, Yang C, Al-Ghouleh A, Tighe P, Jones N, Sewell HF, Shakib F, Martinez-Pomares L, Ghaemmaghami AM. The mannose receptor mediates the uptake of diverse native allergens by dendritic cells and determines allergen-induced T cell polarization through modulation of IDO activity. J Immunol. 2010;185:1522–1531. doi: 10.4049/jimmunol.1000774. [DOI] [PubMed] [Google Scholar]
- 10.Flinterman AE, Pasmans SG, den Hartog Jager CF, Hoekstra MO, Bruijnzeel-Koomen CA, Knol EF, van Hoffen E. T cell responses to major peanut allergens in children with and without peanut allergy. Clin Exp Allergy. 2010;40:590–597. doi: 10.1111/j.1365-2222.2009.03431.x. [DOI] [PubMed] [Google Scholar]
- 11.Prussin C, Lee J, Foster B. Eosinophilic gastrointestinal disease and peanut allergy are alternatively associated with IL-5+ and IL-5(−) T(H)2 responses. J Allergy Clin Immunol. 2009;124:1326–1332. e6. doi: 10.1016/j.jaci.2009.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tordesillas L, Goswami R, Benede S, Grishina G, Dunkin D, Jarvinen KM, Maleki SJ, Sampson HA, Berin MC. Skin exposure promotes a Th2-dependent sensitization to peanut allergens. J Clin Invest. 2014;124:4965–4975. doi: 10.1172/JCI75660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, Shreffler WG, Steele P, Henry KA, Adair M, Francis JM, Durham S, Vickery BP, Zhong X, Burks AW. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009;124:292–300. doi: 10.1016/j.jaci.2009.05.022. 00 e1–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patriarca G, Nucera E, Roncallo C, Pollastrini E, Bartolozzi F, De Pasquale T, Buonomo A, Gasbarrini G, Di Campli C, Schiavino D. Oral desensitizing treatment in food allergy: clinical and immunological results. Aliment Pharmacol Ther. 2003;17:459–465. doi: 10.1046/j.1365-2036.2003.01468.x. [DOI] [PubMed] [Google Scholar]
- 15.Nowak-Wegrzyn A, Albin S. Oral immunotherapy for food allergy: mechanisms and role in management. Clin Exp Allergy. 2014 doi: 10.1111/cea.12382. [DOI] [PubMed] [Google Scholar]
- 16.Syed A, Garcia MA, Lyu SC, Bucayu R, Kohli A, Ishida S, Berglund JP, Tsai M, Maecker H, O'Riordan G, Galli SJ, Nadeau KC. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3) J Allergy Clin Immunol. 2014;133:500–510. doi: 10.1016/j.jaci.2013.12.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shevach EM. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity. 2006;25:195–201. doi: 10.1016/j.immuni.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001;107:891–896. doi: 10.1067/mai.2001.114708. [DOI] [PubMed] [Google Scholar]
- 19.Hulse KE, Reefer AJ, Engelhard VH, Patrie JT, Ziegler SF, Chapman MD, Woodfolk JA. Targeting allergen to FcgammaRI reveals a novel T(H)2 regulatory pathway linked to thymic stromal lymphopoietin receptor. J Allergy Clin Immunol. 2010;125:247–256. e1–e8. doi: 10.1016/j.jaci.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moller BK, Andresen BS, Christensen EI, Petersen CM. Surface membrane CD4 turnover in phorbol ester stimulated T-lymphocytes. Evidence of degradation and increased synthesis. FEBS Lett. 1990;276:59–62. doi: 10.1016/0014-5793(90)80506-e. [DOI] [PubMed] [Google Scholar]
- 21.Mao JH, Chen ZM, Tang YM, Liang L, Du LZ, Zhang Y. Regulation of CD3, CD4 and CD8 expressions on PMA-activated human peripheral T cells. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2004;33:155–159. doi: 10.3785/j.issn.1008-9292.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Baumgarth N, Roederer M. A practical approach to multicolor flow cytometry for immunophenotyping. J Immunol Methods. 2000;243:77–97. doi: 10.1016/s0022-1759(00)00229-5. [DOI] [PubMed] [Google Scholar]
- 23.Maecker HT, Trotter J. Flow cytometry controls, instrument setup, and the determination of positivity. Cytometry A. 2006;69:1037–1042. doi: 10.1002/cyto.a.20333. [DOI] [PubMed] [Google Scholar]
- 24.Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A. 2011;79:167–174. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown SG. Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol. 2004;114:371–376. doi: 10.1016/j.jaci.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 26.Gavin MA, Torgerson TR, Houston E, DeRoos P, Ho WY, Stray-Pedersen A, Ocheltree EL, Greenberg PD, Ochs HD, Rudensky AY. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci U S A. 2006;103:6659–6664. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, Roncarolo MG, Levings MK. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–354. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 28.Pillai V, Ortega SB, Wang CK, Karandikar NJ. Transient regulatory T-cells: a state attained by all activated human T-cells. Clin Immunol. 2007;123:18–29. doi: 10.1016/j.clim.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reefer AJ, Carneiro RM, Custis NJ, Platts-Mills TA, Sung SS, Hammer J, Woodfolk JA. A role for IL-10-mediated HLA-DR7-restricted T cell-dependent events in development of the modified Th2 response to cat allergen. J Immunol. 2004;172:2763–2772. doi: 10.4049/jimmunol.172.5.2763. [DOI] [PubMed] [Google Scholar]
- 30.Woodfolk JA. T-cell responses to allergens. J Allergy Clin Immunol. 2007;119:280–294. doi: 10.1016/j.jaci.2006.11.008. quiz 95–6. [DOI] [PubMed] [Google Scholar]
- 31.Burastero SE. Pollen-cross allergenicity mediated by panallergens: a clue to the patho-genesis of multiple sensitizations. Inflamm Allergy Drug Targets. 2006;5:203–209. doi: 10.2174/187152806779010918. [DOI] [PubMed] [Google Scholar]
- 32.Moran TP, Vickery BP, Burks AW. Oral and sublingual immunotherapy for food allergy: current progress and future directions. Curr Opin Immunol. 2013;25:781–787. doi: 10.1016/j.coi.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wisniewski J, Agrawal R, Woodfolk JA. Mechanisms of tolerance induction in allergic disease: integrating current and emerging concepts. Clin Exp Allergy. 2013;43:164–176. doi: 10.1111/cea.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wambre E, DeLong JH, James EA, LaFond RE, Robinson D, Kwok WW. Differentiation stage determines pathologic and protective allergen-specific CD4+ T-cell outcomes during specific immunotherapy. J Allergy Clin Immunol. 2012;129:544–551. 51, e1–e7. doi: 10.1016/j.jaci.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim BS, Kim IK, Park YJ, Kim YS, Kim YJ, Chang WS, Lee YS, Kweon MN, Chung Y, Kang CY. Conversion of Th2 memory cells into Foxp3+ regulatory T cells suppressing Th2-mediated allergic asthma. Proc Natl Acad Sci U S A. 2010;107:8742–8747. doi: 10.1073/pnas.0911756107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anagnostou K, Islam S, King Y, Foley L, Pasea L, Bond S, Palmer C, Deighton J, Ewan P, Clark A. Assessing the efficacy of oral immunotherapy for the desensitisation of peanut allergy in children (STOP II): a phase 2 randomised controlled trial. Lancet. 2014;383:1297–1304. doi: 10.1016/S0140-6736(13)62301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varshney P, Jones SM, Scurlock AM, Perry TT, Kemper A, Steele P, Hiegel A, Kamilaris J, Carlisle S, Yue X, Kulis M, Pons L, Vickery B, Burks AW. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol. 2011;127:654–660. doi: 10.1016/j.jaci.2010.12.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nozawa A, Okamoto Y, Moverare R, Borres MP, Kurihara K. Monitoring Ara h 1, 2 and 3-sIgE and sIgG4 antibodies in peanut allergic children receiving oral rush immunotherapy. Pediatr Allergy Immunol. 2014;25:323–328. doi: 10.1111/pai.12243. [DOI] [PubMed] [Google Scholar]
- 39.Vickery BP, Lin J, Kulis M, Fu Z, Steele PH, Jones SM, Scurlock AM, Gimenez G, Bardina L, Sampson HA, Burks AW. Peanut oral immunotherapy modifies IgE and IgG4 responses to major peanut allergens. J Allergy Clin Immunol. 2013;131:128–134. e1–e3. doi: 10.1016/j.jaci.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wachholz PA, Durham SR. Mechanisms of immunotherapy: IgG revisited. Curr Opin Allergy Clin Immunol. 2004;4:313–318. doi: 10.1097/01.all.0000136753.35948.c0. [DOI] [PubMed] [Google Scholar]
- 41.Uermosi C, Beerli RR, Bauer M, Manolova V, Dietmeier K, Buser RB, Kundig TM, Saudan P, Bachmann MF. Mechanisms of allergen-specific desensitization. J Allergy Clin Immunol. 2010;126:375–383. doi: 10.1016/j.jaci.2010.05.040. [DOI] [PubMed] [Google Scholar]
- 42.Burton OT, Logsdon SL, Zhou JS, Medina-Tamayo J, Abdel-Gadir A, Noval Rivas M, Koleoglou KJ, Chatila TA, Schneider LC, Rachid R, Umetsu DT, Oettgen HC. Oral immunotherapy induces IgG antibodies that act through FcgammaRIIb to suppress IgE-mediated hypersensitivity. J Allergy Clin Immunol. 2014 doi: 10.1016/j.jaci.2014.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.