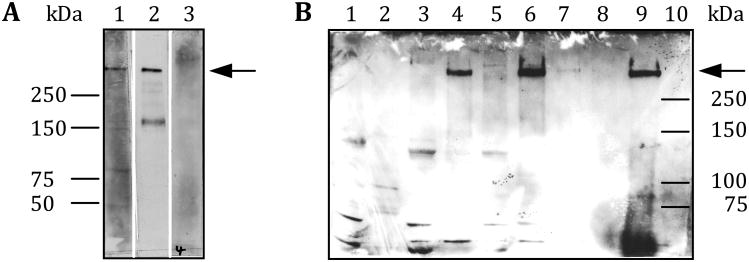
Figure 5. Versican V2 is the IB4-binding splice variant.
A subcellular fraction of rat spinal cord tissue was treated with hyaluronidase to release hyaluronan-associated proteins such as versican into the supernatant. The extract was then either directly analyzed by Western blotting using anti-GAG alpha or GAG beta specific antibodies (A) or first immunoprecipitated with a monoclonal anti-versican antibody and subsequently analyzed by Western blotting using the anti-GAG alpha selective antibody only (B). A) A hyaluronidase extracted subcellular fraction of rat spinal cord tissue only contains V2. Lane 1: Hyaluronidase extract probed with the anti-GAG alpha antibody; Lane 2: Hyaluronidase extract probed with IB4-HRP; Lane 3: Hyaluronidase extract probed with the anti-GAG beta antibody. B) The IB4-binding molecule is versican V2. Proteins derived from the same subcellular fractions as those that were used to demonstrate the enrichment of the IB4-binding molecule in Figure 1 were separated by SDS-PAGE and analyzed by Western blotting with the anti-GAG alpha specific antibody. See fractionation scheme on the right in Fig. 1 for more detailed information about the different subcellular fractions that were analyzed and the amount of protein that was loaded onto each lane. Arrow: Position of the IB4-binding molecule/anti-GAG alpha immunoreactivity on the Western blot.