Abstract
The aim of the study
The aim of the study was to investigate the serum pro-inflammatory cytokine profile in patients with diagnosed endometriosis.
Material and methods
The study included 160 women, who were divided in two study groups (Group I – endometriosis; Group 2 – healthy). We evaluated the serum levels of interleukin (IL)-1β, IL-5, IL-6, IL-7, and IL-12, and of tumour necrosis factor α (TNF-α) with the use of Human Multiplex Cytokine Panels.
Results
The serum level of IL-1β, IL-6, and TNF-α is significantly higher in women with endometriosis compared to women free of disease, from the control group (mean 10.777, 183.027, and 131.326, respectively, compared to 3.039, 70.043, and 75.285, respectively; p = 0.002, p < 0.001, and p = 0.015, respectively). No significant differences in the serum levels of IL-5 and IL-12 were observed between the studied groups, and IL-7 had a very low detection rate.
Conclusions
Women with endometriosis have elevated levels of key pro-inflammatory cytokines, i.e. IL-1β, IL-6, and TNF-α. At the same time, IL-1β and IL-6 could be used as predictors for endometriosis.
Keywords: inflammation, endometriosis, cytokine, interleukin
Introduction
Endometriosis is a pelvic inflammatory condition defined as the presence of ectopic deposits of endometrial tissue outside of the uterine cavity. The disease manifests clinically through various forms of pelvic pain or subfertility. The presence of pelvic endometriosis is about 5-10% in the general population, but in women with pelvic pain, infertility, or both, the prevalence is 35-50% [1], with some authors reporting as high as 82% prevalence in these patients [2]. This variability could be due to the difficulty in non-surgical diagnosis. The gold standard in diagnosing endometriosis is diagnostic laparoscopy. On the other hand, it is an invasive procedure with potential hazards, which can include major vessel or bowel injury [3]. A simple blood test for prediction and diagnosis of endometriosis would overcome these problems and have a major impact on women's health.
Immune system alterations are thought to be involved in the development of endometriosis, especially a dysfunction in immune-related cells and macrophages within the peritoneum secreting a number of products, mainly cytokines and growth factors [4, 5]. At this level, there is an immune-inflammatory reaction that activates immune cells, together with endometriotic implants, producing high amounts of cytokines, growth factors, and angiogenic products [6]. However, systemic immune alterations have also been described in endometriosis, with activation of peripheral blood monocytes, which secrete high levels of cytokines [7]. Several cytokines, including interleukin 6 (IL-6), vascular endothelial growth factor (VEGF), and tumour necrosis factor α (TNF-α), have been studied in the pathogenesis of endometriosis. Interleukin 6 is considered to play a potential role in the growth and/or maintenance of ectopic endometrial tissue. Interleukin 6 is a regulator of inflammation and immunity that modulates secretion of other cytokines, promotes T-cell activation and B-cell differentiation, and inhibits growth of various cell lines. The role of IL-6 in the pathogenesis of endometriosis has been extensively studied, but the levels of IL-6 detected both in the peritoneal fluid and in the serum of patients with endometriosis have been inconsistent [8, 9]. Tumor necrosis factor α is secreted from activated macrophages, and it has potent inflammatory, cytotoxic, and angiogenic effects. It is known to stimulate the expression of matrix metalloproteinases by endometriotic tissues, and matrix metalloproteinases actively participate in the invasion and matrix remodelling of endometriotic lesions [10]. Moreover, etanercept, an anti-TNF therapy, was found to effectively reduce the development of endometriosis [11]. Some authors observed that increased production of these factors in the peritoneal fluid was associated with the elevation of similar factors in the peripheral blood of patients with endometriosis [12].
The present study aimed to investigate the serum pro-inflammatory cytokine profile in patients with endometriosis compared with healthy controls, and to evaluate the sensitivity and specificity of any single cytokine or a combination of cytokines in the prediction of the inflammatory status in endometriosis.
Material and methods
Study population and design
A case-control study was conducted between June 2013 and June 2014 in “Dominic Stanca” Obstetrics and Gynaecology Clinic, Cluj-Napoca, Romania.
The study included 160 patients admitted to the clinic, who were divided into two groups as follows: Group I (endometriosis group) – 80 women with regular menses, and with no history of pelvic infections, autoimmune and neoplastic diseases, undergoing laparoscopy or laparotomy for suspected endometriosis. The evidence of endometriosis was verified by histopathological analysis. The severity of endometriosis was staged according to the revised American Society for Reproductive Medicine (rASRM) classification [13]. Group II (control group) – 80 healthy non-pregnant women aged between 18 and 40 years old, without clinical and paraclinical evidence of endometriosis.
Exclusion criteria were as follows: previous pelvic surgeries, history of cancer, suspected malignancy, adenomyosis or leiomyoma, pre-surgical suspicion of evidence of premature ovarian failure, or the use of ovarian suppressive drugs, such as oral contraceptives, GnRH agonists, progestins, or danazol in the preceding six months. None of the patients had taken anti-inflammatory medications or had been diagnosed with an inflammatory or infectious condition for ≥ 6 months before the study.
The study protocol was approved by the Local Ethics Committee of “Iuliu Haţieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania, and signed informed consent was received from each woman before sample collection. The study was conducted under the tenets of the Helsinki Declaration.
Data was collected for each subject included in the study in a form containing general and anthropometric data (weight, height), the heredo-collateral history, personal pathological history, and data on the age and onset of symptoms. The body mass index (BMI) was calculated as the ratio between the weight (kg) and the squared height (in metres). A total of 5 ml of venous blood was collected from each patient before breakfast, which was centrifuged, and the serum obtained was stored at –70°C for future determinations.
Cytokine evaluation
We used multiplex cytokine kits (Invitrogen Human Cytokine 30-Plex Panel, LHC6003) in order to measure serum levels of IL-1β, IL-5, IL-6, IL-7, IL-12, and TNF-α. Dose measurements were performed with the use of a Luminex 200 system (Luminex Corporation, Austin, TX, USA) in accordance with the manufacturer's specifications (Invitrogen Corporation, Carlsbad, CA, USA). The sensitivity of the test was specified by the manufacturer (Invitrogen Corporation, Carlsbad, CA, USA) in the informative material included in the kits.
The average sensitivity of the test for IL-1β was 5 pg/ ml, with an inter-assay variation coefficient of 4.8%. For IL-5, the average sensitivity of the test was 0.5 pg/ml with an inter-assay variation coefficient of 7.5%. The average sensitivity of the test for IL-6 was 0.5 pg/ml with an inter-assay variation coefficient of 7%. In the case of IL-7, the average sensitivity of the test was 10 pg/ml with an inter-assay variation coefficient of 9.8%. The sensitivity of the test for IL-12 was 1 pg/ml, and the inter-assay variation coefficient of 8.1%. The test for TNF-α revealed an average sensitivity of 0.5 pg/ml, with inter-assay variation coefficient of 8.3%.
Statistical analysis
Statistical analyses were performed using Microsoft Excel and IBM SPSS software (version 22.0). Data is presented as median and quartiles for the groups because the standard deviation was of importance. We performed an analysis for the significances of the observed differences with the median test for independent samples; also Pearson's χ2 test with and without the continuity correction, and Fisher exact test were used as statistical tests. P values less than 0.05 were regarded as significant. Because the study we had conducted was a case-control study, the predictive values (PPV – positive predictive value and NPV – negative predictive value) were calculated with the Bayesian approximation, assuming an endometriosis prevalence of 10%.
Results
Tables 1 and 2 present the biometry data and inflammatory markers considered for the study. Almost all of the cases from the endometriosis group were staged as III (30 – 37.50%) or IV (47 – 58.75%), and only 3 cases (3.75%) were in stage II of endometriosis, according to rASRM staging criteria.
Table 1.
Descriptive statistics of the endometriosis and control groups
| Variable | Calculated parameters | Endometriosis group | Control group | Average |
|---|---|---|---|---|
| Age (yr) | mean ± SD | 30.600 ±5.486 | 26.350 ±2.131 | 28.475 ±4.655 |
| Weight (kg) | mean ± SD | 62.050 ±9.067 | 56.925 ±8.094 | 59.488 ±8.920 |
| Height (cm) | mean ± SD | 164.725 ±5.114 | 167.225 ±6.773 | 165.975 ±6.094 |
| BMI (kg/cm2) | mean ± SD | 22.912 ±3.520 | 20.307 ±2.126 | 21.609 ±3.173 |
Table 2.
Descriptive statistic of the studied pro-inflammatory parameters
| Parameter | IL-1β | IL-5 | IL-6 | IL-12 | TNF-α | |
|---|---|---|---|---|---|---|
| N | valid | 110 | 38 | 146 | 136 | 146 |
| missing | 50 | 122 | 4 | 24 | 14 | |
| median | 4.170 | 0.035 | 69.052 | 2.080 | 88.552 | |
| minimum | 0.0 | 0.0 | 11.3 | 0.2 | 0.5 | |
| maximum | 53.2 | 0.5 | 542.3 | 16.1 | 584.5 | |
| percentiles | 25 | 0.363 | 0.035 | 61.854 | 1.325 | 26.704 |
| 50 | 4.170 | 0.035 | 69.052 | 2.080 | 88.552 | |
| 75 | 10.325 | 0.071 | 144.192 | 4.180 | 143.325 |
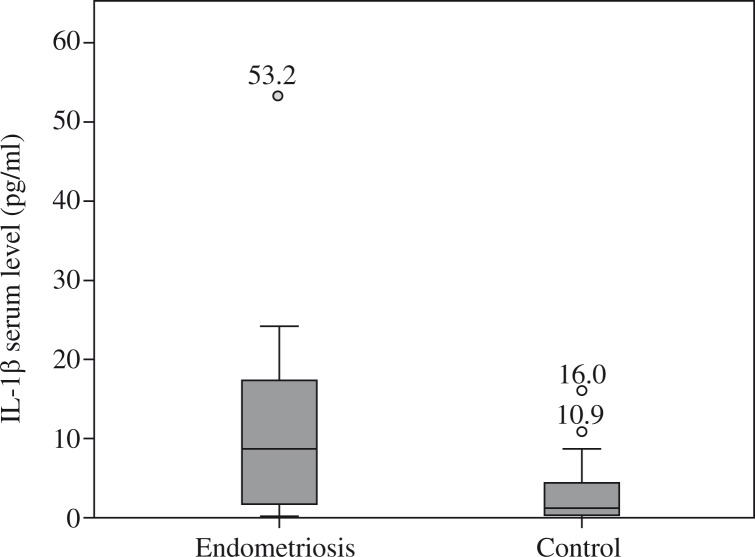
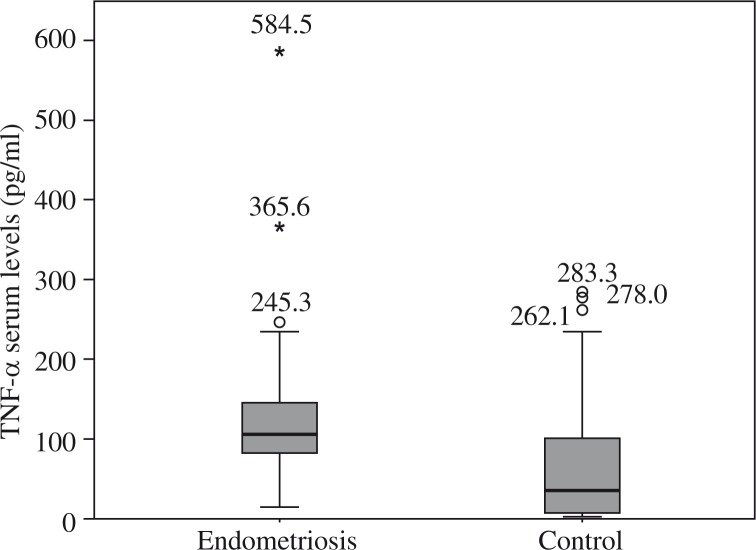
Table 3 presents the data obtained by inferential statistic (the independent samples median-test probability), which show that mean serum levels of IL-1β, IL-6, and TNF-α were significantly higher in patients with endometriosis compared to healthy controls (mean 10.777, 183.027, and 131.326, respectively, compared to 3.039, 70.043, and 75.285, respectively; p = 0.002, p < 0.001, and p = 0.015, respectively).
Table 3.
Inferential statistic for the studied markers
| Independent samples median-test probability* | |
|---|---|
| IL-1β | 0.010 |
| IL-5 1 | (using Fisher exact test) |
| IL-6 | < 0.001 |
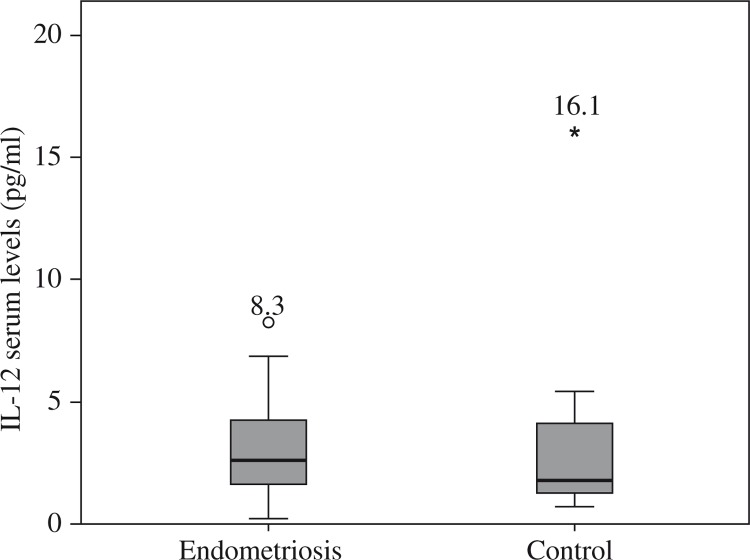
| IL-12 | 0.467 |
| TNF-α | 0.026 |
p < 0.05 significant difference compared to control group
Figures 1–5 show the distribution and median of the studied markers’ serum levels between the groups with a significantly higher serum level of IL-1β, IL-6 and TNF-α in the endometriosis group, and no significant differences in the serum levels of IL-5 and IL-12 between the studied groups. Interleukin 7 had a very low detection rate.
Fig. 1.
Distribution of interleukin 1β serum levels among groups
Fig. 5.
Distribution of tumor necrosis factor α serum levels among groups
Fig. 2.
Distribution of interleukin 5 serum levels among Groups
Fig. 3.
Distribution of interleukin 6 serum levels among Groups
Fig. 4.
Distribution of interleukin 12 serum levels among Groups
Because the independent samples median test showed significant differences between the median serum levels of IL-1β, IL-6, and TNF-α in endometriosis and control groups, we have also evaluated the use of these markers as a predictive factor for endometriosis. Threshold values chosen for the three parameters (IL-1β – 7 pg/ml, IL-6 – 125 pg/ml, and TNF-α – 100 pg/ml) have minimised overlapping confidence intervals from the observed distribution. We have divided the studied groups according to the three new tests as positives and negatives.
Tables 4 and 5 show the results obtained for IL-1β and IL-6 as a predictor of endometriosis, with statistically significant results. Pearson's χ2 test validated the observed differences, with a probability of 0.005 for IL-1β and < 0.001 for IL-6 (with the continuity correction probability was < 0.001). Table 6 shows the results obtained for TNF-α as a predictive test for endometriosis, showing a lack of statistical significance. Pearson's χ2 test failed to validate the observed differences, with a probability of 0.074 (the probability with the continuity correction was also 0.072).
Table 4.
Contingency table for interleukin 1β as the golden standard
| Endometriosis group | Control group | Total | |
|---|---|---|---|
| Interleukin β positive | 32 | 8 | 40 |
| Interleukin 1β negative | 24 | 46 | 70 |
| Total | 56 | 54 | 110 |
| Sensitivity | 0.571 | ||
| Specificity | 0.851 | ||
| Positive predictive value* | 0.300 | ||
| Negative predictive value* | 0.947 | ||
Calculated with the Bayesian approximation for case-control studies, assuming a endometriosis prevalence of 10%
Table 5.
Contingency table for interleukin 6 as the golden standard
| Endometriosis group | Control group | Total | |
|---|---|---|---|
| Interleukin 6 positive | 38 | 4 | 42 |
| Interleukin 6 negative | 38 | 76 | 114 |
| Total | 76 | 80 | 156 |
| Sensitivity | 0.500 | ||
| Specificity | 0.950 | ||
| Positive predictive value* | 0.526 | ||
| Negative predictive value* | 0.945 | ||
Calculated with the Bayesian approximation for case-control studies, assuming a endometriosis prevalence of 10%
Table 6.
Contingency table for tumor necrosis factor (TNF-α) as the golden standard
| Endometriosis group | Control group | Total | |
|---|---|---|---|
| TNF-α positive | 38 | 20 | 58 |
| TNF-α negative | 34 | 54 | 88 |
| Total | 72 | 74 | 146 |
| Sensitivity | 0.527 | ||
| Specificity | 0.729 | ||
| Positive predictive value* | 0.178 | ||
| Negative predictive value* | 0.933 | ||
Calculated with the Bayesian approximation for case-control studies, assuming a endometriosis prevalence of 10%
Regarding staging of the endometriosis group, no significant differences were observed in the means or median values of IL-1β, IL-5, IL-6, IL-12, and TNF-α if the stage of endometriosis was used as a grouping parameter, between patients with endometriosis stage III and stage IV (Table 7).
Table 7.
Endometriosis stages III and IV comparison
| Independent samples median-test probability* | |
|---|---|
| Interleukin 1β | 1 (using Fisher exact test) |
| Interleukin 5 | 1 (using Fisher exact test) |
| Interleukin 6 | 0.892 |
| Interleukin 12 | 0.839 |
| TNF-α | 0.591 |
p < 0.05 significant difference compared to control group
Discussion
In the present study we found that in women with endometriosis there is a significantly higher serum level of IL-1β, IL-6, and TNF-α, compared to healthy controls. We also found that IL-5 and IL-12 did not differ significantly between women with endometriosis and women free of the disease. On the other hand, IL-7 had a very low detection rate in the studied groups, so we were unable to draw any conclusions regarding its implication.
At this moment, there are a large number of studies that support the pathophysiological implication of pro-inflammatory and anti-inflammatory cytokines such as IL-2, IL-4, IL-6, IL-8, IL-10, interferon γ (IFN-γ), and TNF-α in endometriosis [14, 15].
Interleukin 6 is a cytokine derived from T cells, secreted by macrophages, lymphocytes, fibroblasts, and endothelial cells. Its secretion is increased by peritoneal and macrophages in the case of endometriosis [16]. Generally, IL-6 inhibits the growth of endometrial cells, the effect of which seems to be lost in endometriotic tissues [17]. Cytokines, and specifically IL-6, have been investigated regarding the pathogenesis of endometriosis, and also as a predictor of the disease [18, 19]. Elevated serum CA-125 and IL-6 are considered to be biological markers for differential diagnosis in endometriosis [20]. Previous studies found conflicting results regarding serum levels of IL-6 in endometriosis; however, most of them were in favour of a significant increase in IL-6 serum or peritoneal fluid (PF) levels in the case of endometriosis. Anterior reports [21, 22] showed significantly higher IL-6 levels in early stages of endometriosis as compared to the healthy group. Moreover, one study showed a correlation between IL-6 and pain, as well as a relation between cytokine expression and recurrences of endometriosis [23]. On the other hand, a recent study showed no statistically significant difference in serum IL-6 concentration between subjects with endometriosis and controls; consequently, the authors do not recommend measuring IL-6 in the serum as a predictor of endometriosis. The same authors also suggested that IL-6 provides the best discrimination between subjects with endometriosis and healthy controls, and the diagnostic value of this marker increased with the finding that serum IL-6 levels did not change significantly during any phase of the menstrual cycle in either group [3, 24]. Other similar studies have shown a positive relationship between PF levels of IL-6 with severity of endometriosis [25, 26].
Tumor necrosis factor α is a pro-inflammatory cytokine produced mainly by activated macrophages. It promotes the production of other pro-inflammatory cytokines, such as IL-1, IL-6, and additional TNF-α. It is involved in the normal physiology of endometrial proliferation and shedding [27]. It was shown that peritoneal fluid TNF-α concentrations are elevated in women with endometriosis, and some studies showed that higher concentrations correlate with the stage of disease. One study observed that serum TNF-α levels and urinary sFlt-1 levels corrected for creatinine excretion were significantly increased in the endometriosis group [28]. Tumor necrosis factor α may play a central role in the local and systematic manifestations of endometriosis, on the basis of evidence showing that it promotes the growth of endometriotic cells. Moreover, some studies explored the association of TNF-α gene polymorphisms and endometriosis, and it seems that some polymorphisms are involved in the pathogenesis of endometriosis [29, 30]. On the other hand, blocking TNF-α appears to inhibit the development of the disease in animal models [27].
Interleukin 1 is a cytokine that plays an important role in inflammation and immune response. The IL-1 family consists of IL-1α, IL-1β, and IL-1 receptor antagonist. Both IL-1α and IL-1β are the most potent pro-inflammatory cytokines, and IL-1 receptor antagonist is a naturally occurring anti-inflammatory cytokine [31, 32]. A series of studies showed that there are increased concentrations of IL-1β, IL-6, IL-10, and TNF-α, as well as decreased VEGF in the folicular fluid (FF) of endometriosis patients [33, 34]. Moreover, Lambert et al. have shown both a significant increase in serum IL-1β and IL-1sRII levels in deep infiltrating endometriosis (DIE) compared to superficial endometriosis (SUP) and normal women, and suggested that a defect in the control of IL-1 can impact the pathophysiology of endometriosis [35]. On the other hand, Kalu et al. [36] found no significant difference in serum levels of platelet-derived growth factor (PDGF), IL-6, Regulated on Activation, Normal T Cell Expressed and Secreted chemokine (RANTES), IL-1β, TNF-α, and sFas in patients with endometriosis, compared to controls.
Applying IL-1β and IL-6 for the prediction of endometriosis, we found that they can be successfully used, having a specificity of 0.85, respectively, and a specificity of 0.95. Because IL-1β is not very sensitive (0.57), it is recommended for use in conjunction with other tests. Tumor necrosis factor α, used in the prediction of endometriosis, has limited value with a specificity of 0.72.
Our results are in accordance with a recent study, which reported increased peritoneal concentrations of IL-6 and IL-10 in women with endometriosis. Analysis of the concentrations of these cytokines showed differential diagnostic usefulness for endometriosis. Peritoneal concentrations of IL-6 offer relatively good diagnostic accuracy [37]. At the same time, a very recent study has shown that at the cut-off value of 3.00 pg/ml peritoneal TNF-α can be a reliable screening marker for the prediction of endometriosis in adolescents [38]. In contrast with our observations, Bedaiwy et al. [12] failed to show the significance of peritoneal IL-6 levels in diagnosing women with endometriosis.
One of our main limitations is represented by the study population, which are only Caucasian women. Most of the studies present in the literature had mainly Asian origin subjects, this being considered a possible bias mark [39]. From this point of view, our population could be considered also a strength, but a more heterogenic study group would probably give more accurate results. Another limitation could be the fact that most of the patients included in the study were stage III or IV (96.25%) and the lack of differentiation between patients with ovarian endometriomas (OE) and patients with DIE. As the study was conducted in a University Clinic, the patients addressing for treatment and included in the study had late stages of endometriosis, thus the low rate of early stages. Probably future studies could include patients presenting for unexplained infertility undergoing exploratory laparoscopy, and thus including patients with early stages discovered incidentally. On the other hand, OE and DIE are considered two distinct entities of endometriotic disease, and accordingly it is accepted that endometriosis progresses to cystic ovarian disease and pelvic adhesions in some women, to deeply infiltrating disease in other women, and sometimes to both stages of severe disease in the same woman [5]. A recent study has found a high positive correlation between serum levels of IL-6 and IL-8 in patients with OE, but not in the DIE and control groups [7]. One last limitation could be represented by the severity of endometriosis in the study population. Most of the recruited patients had advanced endometriosis, OE, or DIE, and consequently a differentiation in serum levels of the studied markers between patients with SUP and OE or DIE was not possible. We could also take in consideration the detection sensitivity of multiplexed immunoassays. It was shown that, while multiplexed immunoassays have sensitivity comparable to conventional ELISA, it is possible that the robustness may vary among different multiplex bead arrays [40].
In conclusion, we examined the possible differences in pro-inflammatory cytokine serum levels from patients with endometriosis compared to healthy controls using a multiplexed cytokine assay. We have shown that IL-1β, IL-6, and TNF-α serum levels are significantly higher in women with endometriosis compared to women free of disease, from the control group, and that there is no difference in the serum levels of IL-5 and IL-12 between these patients. More importantly, our study showed that IL-1β and IL-6 could be used as a non-surgical diagnostic test for endometriosis. Further studies are necessary to clarify and confirm the role of pro-inflammatory cytokines in the pathogenesis of endometriosis, and moreover to find a suitable predictive model for early diagnosis.
The authors declare no conflict of interest.
This paper was published under the frame of the European Social Fund, Human Resources Development Operational Programme 2007-2013, project no. POSDRU/159/1.5/S/138776.
References
- 1.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 2.Mounsey A, Wilgus A, Slawson DC. Diagnosis and management of endometriosis. Am Fam Physician. 2006;74:594, 600. [PubMed] [Google Scholar]
- 3.Othman Eel-D, Hornung D, Salem HT, et al. Serum cytokines as biomarkers for nonsurgical prediction of endometriosis. Eur J Obstet Gynecol Reprod Biol. 2008;137:240–246. doi: 10.1016/j.ejogrb.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Yagmur E, Bastu E, Karamustafaoglu-Balci B, et al. Non-invasive diagnosis of endometriosis based on a combined analysis of four plasma biomarkers. Centr Eur J Immunol. 2013;38:154–158. [Google Scholar]
- 5.Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 6.Kubatova A, Erdem A, Erdem M, et al. Serum cytokine and growth factor levels in patients with endometriosis. Centr Eur J Immunol. 2013;38:500–504. [Google Scholar]
- 7.Carmona F, Chapron C, Martínez-Zamora MA, et al. Ovarian endometrioma but not deep infiltrating endometriosis is associated with increased serum levels of interleukin-8 and interleukin-6. J Reprod Immunol. 2012;95:80–86. doi: 10.1016/j.jri.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Moini A, Malekzadeh F, Amirchaghmaghi E, et al. Risk factors associated with endometriosis among infertile Iranian women. Arch Med Sci. 2013;9:506–514. doi: 10.5114/aoms.2013.35420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agic A, Xu H, Finas D, et al. Is endometriosis associated with systemic subclinical inflammation? Gynecol Obstet Invest. 2006;62:139–147. doi: 10.1159/000093121. [DOI] [PubMed] [Google Scholar]
- 10.Klllc SH, Evsen S, Tasdemir N, et al. Follicular fluid vascular endothelial growth factor and tumour necrosis factor a concentration in patients with endometriosis undergoing ICSI. Reprod Biomed Online. 2007;15:316–320. doi: 10.1016/s1472-6483(10)60345-8. [DOI] [PubMed] [Google Scholar]
- 11.Zulfikaroglu E, Klllc S, Islimye M, et al. Efficacy of anti-tumor necrosis factor therapy on endometriosis in an experimental rat model. Arch Gynecol Obstet. 2011;283:799–804. doi: 10.1007/s00404-010-1434-0. [DOI] [PubMed] [Google Scholar]
- 12.Bedaiwy MA, Falcone T, Sharma RK, et al. Prediction of endometriosis with serum and peritoneal fluid markers: a prospective controlled trial. Hum Reprod. 2002;17:426–431. doi: 10.1093/humrep/17.2.426. [DOI] [PubMed] [Google Scholar]
- 13.Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817–821. doi: 10.1016/s0015-0282(97)81391-x. [DOI] [PubMed] [Google Scholar]
- 14.Wu MY, Ho HN. The role of cytokines in endometriosis. Am J Reprod Immunol. 2003;49:285–296. doi: 10.1034/j.1600-0897.2003.01207.x. [DOI] [PubMed] [Google Scholar]
- 15.OuYang Z, Hirota Y, Osuga Y, et al. Interleukin-4 stimulates proliferation of endometriotic stromal cells. Am J Pathol. 2008;173:463–469. doi: 10.2353/ajpath.2008.071044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berkkanoglu M, Arici A. Immunology and endometriosis. Am J Reprod Immunol. 2003;50:48–59. doi: 10.1034/j.1600-0897.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- 17.Dmowski WP, Braun DP. Immunology of endometriosis. Best Pract Res Clin Obstet Gynaecol. 2004;18:245–263. doi: 10.1016/j.bpobgyn.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Bedaiwy MA, Falcone T. Laboratory testing for endometriosis. Clin Chim Acta. 2004;340:41–56. doi: 10.1016/j.cccn.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 19.Ohata Y, Harada T, Miyakoda H, et al. Serum interleukin-8 levels are elevated in patients with ovarian endometrioma. Fertil Steril. 2008;90:994–999. doi: 10.1016/j.fertnstert.2007.07.1355. [DOI] [PubMed] [Google Scholar]
- 20.Martínez S, Garrido N, Coperias JL, et al. Serum interleukin-6 levels are elevated in women with minimal-mild endometriosis. Hum Reprod. 2007;22:836–842. doi: 10.1093/humrep/del419. [DOI] [PubMed] [Google Scholar]
- 21.Mier-Cabrera J, Jiménez-Zamudio L, García-Latorre E, et al. Quantitative and qualitative peritoneal immune profiles, T-cell apoptosis and oxidative stress-associated characteristics in women with minimal and mild endometriosis. BJOG. 2011;118:6–16. doi: 10.1111/j.1471-0528.2010.02777.x. [DOI] [PubMed] [Google Scholar]
- 22.Khan KN, Masuzaki H, Fujishita A, et al. Higher activity by opaque endometriotic lesions than nonopaque lesions. Acta Obstet Gynecol Scand. 2004;83:375–382. doi: 10.1111/j.0001-6349.2004.00229.x. [DOI] [PubMed] [Google Scholar]
- 23.Velasco I, Acién P, Campos A, et al. Interleukin-6 and other soluble factors in peritoneal fluid and endometriomas and their relation to pain and aromatase expression. J Reprod Immunol. 2010;84:199–205. doi: 10.1016/j.jri.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Somigliana E, Vigano P, Tirelli AS, et al. Use of the concomitant serum dosage of CA 125, CA 19-9 and interleukin-6 to detect the presence of endometriosis. Results from a series of reproductive age women undergoing laparoscopic surgery for benign gynaecological conditions. Hum Reprod. 2004;19:1871–1876. doi: 10.1093/humrep/deh312. [DOI] [PubMed] [Google Scholar]
- 25.Milewski Ł, Barcz E, Dziunycz P, et al. Association of leptin with inflammatory cytokines and lymphocyte subpopulations in peritoneal fluid of patients with endometriosis. J Reprod Immunol. 2008;79:111–117. doi: 10.1016/j.jri.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Milewski Ł, Dziunycz P, Barcz E, et al. Increased levels of human neutrophil peptides 1, 2, and 3 in peritoneal fluid of patients with endometriosis: association with neutrophils, T cells and IL-8. J Reprod Immunol. 2001;91:64–70. doi: 10.1016/j.jri.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Lu D, Song H, Shi G. Anti-TNF-α treatment for pelvic pain associated with endometriosis. Cochrane Database Syst Rev. 2013;3:CD008088. doi: 10.1002/14651858.CD008088.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho SH, Oh YJ, Nam A, et al. Evaluation of serum and urinary angiogenic factors in patients with endometriosis. Am J Reprod Immunol. 2007;58:497–504. doi: 10.1111/j.1600-0897.2007.00535.x. [DOI] [PubMed] [Google Scholar]
- 29.Lee GH, Choi YM, Kim SH, et al. Association of tumor necrosis factor-a gene polymorphisms with advanced stage endometriosis. Human Reproduction. 2008;23:977–981. doi: 10.1093/humrep/den016. [DOI] [PubMed] [Google Scholar]
- 30.Teramoto M, Kitawaki J, Koshiba H, et al. Genetic contribution of tumor necrosis factor (TNF)-alpha gene promoter (-1031,-863 and -857) and TNF receptor 2 gene polymorphisms in endometriosis susceptibility. Am J Reprod Immunol. 2004;51:352–357. doi: 10.1111/j.1600-0897.2004.00168.x. [DOI] [PubMed] [Google Scholar]
- 31.Rogus J, Beck JD, Offenbacher S, et al. IL1B gene promoter haplotype pairs predict clinical levels of interleukin-1beta and C-reactive protein. Hum Genet. 2008;123:387–398. doi: 10.1007/s00439-008-0488-6. [DOI] [PubMed] [Google Scholar]
- 32.Singh H, Sachan R, Goel H, Mittal B. Genetic variants of interleukin-1RN and interleukin-1b genes and risk of cervical cancer. BJOG. 2008;115:633–638. doi: 10.1111/j.1471-0528.2007.01655.x. [DOI] [PubMed] [Google Scholar]
- 33.Wunder DM, Mueller MD, Birkhauser MH, Bersinger NA. Increased ENA-78 in the follicular fluid of patients with endometriosis. Acta Obstet Gynecol Scand. 2006;85:336–342. doi: 10.1080/00016340500501715. [DOI] [PubMed] [Google Scholar]
- 34.Gupta S, Goldberg JM, Aziz N, et al. Pathogenic mechanisms in endometriosis-associated infertility. Fertil Steril. 2008;90:247–257. doi: 10.1016/j.fertnstert.2008.02.093. [DOI] [PubMed] [Google Scholar]
- 35.Lambert S, Santulli P, Chouzenoux S, et al. Endometriosis: Increasing concentrations of serum interleukin-1β and interleukin-1sRII is associated with the deep form of this pathology. J Gynecol Obstet Biol Reprod (Paris) 2014;43:735–743. doi: 10.1016/j.jgyn.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 36.Kalu E, Sumar N, Giannopoulos T, et al. Cytokine profiles in serum and peritoneal fluid from infertile women with and without endometriosis. J Obstet Gynaecol Res. 2007;33:490–495. doi: 10.1111/j.1447-0756.2007.00569.x. [DOI] [PubMed] [Google Scholar]
- 37.Wickiewicz D, Chrobak A, Gmyrek GB, et al. Diagnostic accuracy of interleukin-6 levels in peritoneal fluid for detection of endometriosis. Arch Gynecol Obstet. 2013;288:805–814. doi: 10.1007/s00404-013-2828-6. [DOI] [PubMed] [Google Scholar]
- 38.Drosdzol-Cop A, Skrzypulec-Plinta V. Selected cytokines and glycodelin A levels in serum and peritoneal fluid in girls with endometriosis. J Obstet Gynaecol Res. 2012;38:1245–1253. doi: 10.1111/j.1447-0756.2012.01860.x. [DOI] [PubMed] [Google Scholar]
- 39.Clayton DG, Walker NM, Smyth DJ, et al. Population structure, differential bias and genomic control in a large-scale, case-control association study. Nat Genet. 2005;37:1243–1246. doi: 10.1038/ng1653. [DOI] [PubMed] [Google Scholar]
- 40.Elshal MF, McCoy JP. Multiplex bead array assays: performance evaluation and comparison of sensitivity to ELISA. Methods. 2006;38:317–323. doi: 10.1016/j.ymeth.2005.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]